Translate this page into:
Choosing Wisely – Implication based on Indian data in our patients with breast cancer (INR vs. USD)

*Corresponding author: Ajay Bapna, Department of Medical Oncology, Bhagwan Mahaveer Cancer Hospital and Research Center, Malviya Nagar, Jaipur - 302 017, Rajasthan, India. drabapna@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Bapna A, Patni N, Patni S. Choosing Wisely – Implication based on Indian data in our patients with breast cancer (INR vs. USD). Int J Mol Immuno Oncol 2021;6(1):6-10.
Abstract
Objectives:
Breast cancer is increasing in India due to aging population, better awareness among general public, willingness to seek treatment of cancers, and easier access to cancers centers. We present our single-center data over a 2-year period and discuss cost implications taking the example of metronomic chemotherapy maintenance and predictive markers in early breast cancer.
Material and Methods:
Prospectively collected data of all consecutive patients with breast cancer registered between September 2017 and August 2019 were evaluated. Clinical features, stage, receptor status, and other features were tabulated. Statistical analysis was using SAS version 9.4 – Chi-square test and Fisher’s exact test were performed. P ≤ 0.05 was considered as statistically significant.
Results:
For the 484 consecutive patients, the median age was 50 years. This included EBC (201, 42%), LABC (141, 29%), and MBC (142, 29%). ER expression was seen in 52% of cases (253/484), PR in 47% (229/484), and Her2 was positive in 47% (229/484). Finally, 83 patients (17%) were identified as TNBC. HR-positive Her2-negative EBC constituted 111/484 patients (23%).
Discussion:
If our 83 TNBC patients were given metronomic maintenance chemotherapy, their 3-year overall survival (OS) is projected to increase from 54% to 100% at a cost of INR 8191/- per patient (equivalent to USD 109/-). If our 111 HR-positive Her2-negative EBC patients were evaluated for risk by biomarker test validated in Indian patients, 76 of these would be spared the toxicity of adjuvant CT. This would also result in saving on the cost of chemotherapy medication of INR 4,035,296/- in India (equivalent to USD 53,699/- if treated in USD). In addition, they would also have better quality of life (QoL).
Conclusion:
It is possible to identify patients with low risk early breast cancer using Can assist and save them from unnecessary cost and/or toxicity.
Keywords
Cost implications
Toxicity
Overtreatment
Metronomic chemotherapy
Maintenance
INTRODUCTION
Patients with breast cancer are increasing in India (like other parts of the world) due to a combination of various factors – actual increase in the incidence, aging population, better awareness among general public, willingness to seek treatment of cancers, and easier access to cancers centers.[1,2] We have previously published our data on 370 consecutive patients with breast cancer seen at our center from August 2015–2017.[3] We now present our data for the subsequent 2-year period and discuss how we can wisely use published Indian data to choose the right treatment option in our patients with breast cancer. We have then taken Indian publications to evaluate cost implications of metronomic chemotherapy maintenance in triple negative breast cancer (TNBC) and predictive biomarker testing to avoid chemotherapy in low-risk hormone receptor (HR)-positive Her2/neu (Her2)-negative early breast cancer (EBC).
MATERIAL AND METHODS
Prospectively collected data of all consecutive patients with breast cancer of infiltrating duct carcinoma (IDC) type at the Bhagwan Mahaveer Cancer Hospital and Research Center, Jaipur (Rajasthan), registered between September 2017 and August 2019 were evaluated, retrospectively. Data regarding age, menopausal status and lymph node positivity, and site of tumor were specifically focused upon. Tumor biopsy reports of estrogen receptor, progesterone receptor, and Her2 testing were also tabulated. It was our hospital’s policy to do Her2 testing by immunohistochemistry (IHC) first and fluorescent in situ hybridization (FISH) was done only when necessary.
American tumor-node-metastasis (AJCC TNM) staging seventh edition was used to divide patients into early breast cancer (EBC; Stage I, IIA, or IIB disease with T2N1), locally advanced breast cancer (LABC; Stage IIB with T3N0, IIIA or IIIC), and metastatic breast cancer (MBC; Stage IV).[4]
Patients were also divided into two age groups, those 50 of age or younger and those above the age of 50 years. Data were statistically analyzed using SAS version 9.4. For categorical data, “Chi-square test” and “Fisher’s exact test” were performed. P ≤ 0.05 was considered as statistically significant.
RESULTS
There were a total of 484 consecutive breast cancer patients diagnosed at our center over the 24-month period between September 2017 and August 2019. The median age was 50 years with a range from 26 to 88 years (256 patients 50 years or younger; 228 above the age of 50 years). Premenopausal patients numbered 171 and 313 were postmenopausal. Correlation between age and menopausal status is shown in Table 1.
| Pre menopausal |
Post menopausal |
Total | |
|---|---|---|---|
| Age ≤ 50 years | 169 | 87 | 256 |
| Age > 50 years | 2 | 226 | 228 |
| Total | 171 | 313 | 484 |
The right-sided breast cancer was diagnosed in 49% (236/484) of patients and the tumor was on the left side in 51% (245/484). Interestingly, three patients presented with bilateral breast cancer.
At the end of the diagnostic investigations, patients were divided into EBC (201, 42%), LABC (141, 29%), and MBC (142, 29%) using the 7th AJCC breast cancer stating criteria.[5]
The axillary lymph node (LN) status of the 342 nonmetastatic (EBC + LABC) is shown in Table 2. A total of 108 patients (47%) did not have any axillary LN involvement. Another 108 had 1 to 4 axillary LN showing metastasis. The remaining 73 patients had more than 4 axillary LNs involved by the cancer.
| Axillary LN involvement |
n = | % | Age range | Age median |
|---|---|---|---|---|
| Zero | 161 | 47.08 % | 30 to 88 | 52 |
| 1 to 4 | 108 | 31.58 % | 26 to 80 | 50 |
| > 4 | 73 | 21.34 % | 28 to 78 | 48 |
| Total cases | 342 | 100 % | ||
ER expression was seen in 52% of cases (253/484), PR in 47% (229/484), and Her2 was positive in 47% (229/484) [Figure 1]. For the 26 patients whose Her2 status was equivocal by immunohistochemistry (IHC), fluorescent in situ hybridization (FISH) was able to clearly identify 23 as Her2 negative and the remaining 3 as Her2 positive.
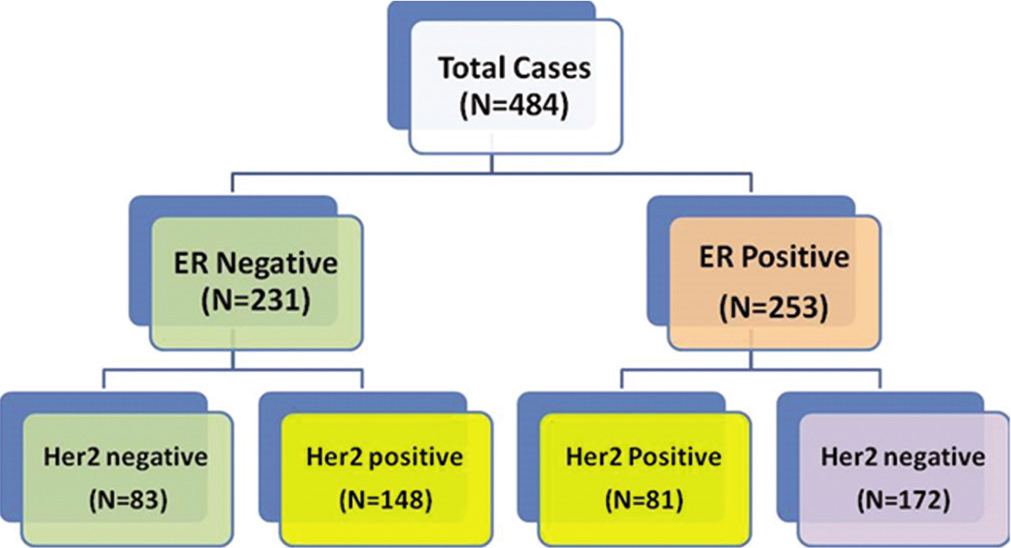
- ER and her 2 status.
Finally, 83 patients (17%) were identified as TNBC [Table 3]. Their median age was 46 years and 59% (49/83) were aged 50 years or lower. Of these 83 TNBC, 38 did not have involvement of the axillary LNs.
| Age | |
|---|---|
| Median | 46 years |
| Range | 29 to 78 years |
| ≤ 50 yrs | 49 (59 %) |
| > 50 yrs | 44 (41 %) |
| Axillary LN Status | |
| Negative | 38 |
| Positive | 45 |
Among the 201 EBC patients, 111 (55%) were ER positive as well as Her2 negative [Figure 2].
- Selected features of ER +ve, Her 2 −ve EBC patients.
DISCUSSION
Our patients with breast cancer have not changed much over the 4-year period from 2015 to 2019 [Table 4]. This would indicate that breast cancer pattern has remained stable at our center.[3] However, cost of treatment remains a continuing challenge, in India and globally.[6,7] Let us take the example of trastuzumab. In India, its use for 9 weeks is cost effective but not for 1 year (at the current price).[6] And globally, efforts to find evidence that shorter durations of trastuzumab are not inferior continue.[7]
| 2015–17 | 2017–19 | |
|---|---|---|
| Age | ||
| 40 years or less | 21 % | 17 |
| 41–60 years | 61 % | 64 |
| More than 60 years | 18 % | 19 |
| N = | 470 | 484 |
| ER positive | 50 % | 52 |
| PR positive | 47 % | 47 |
| Her2 positive | 50 % | 48 |
| TNBC | 17 % | 17 |
TNBC
Our incidence of TNBC (17%) is similar to that reported from the west (15%) and contrary to other reports from India.[8]
TNBC is said to be a disease with poor prognosis, suboptimal response to therapy and therefore is a disease with unmet needs.[9-11]
Various biomarkers such as circulating tumor cells, cell-free DNA, and PD-L1 expression as well as novel therapeutic agents have been studied.[12-15]
Till they become standard of care, is there a way of improving the survival in patients with TNBC? Banavali et al. studied the role of maintenance metronomic chemotherapy in a prospective randomized study in Indian patients with TNBC.[16]
All patients received six cycles of standard cyclophosphamide, adriamycin, and 5-fluouracil (CAF). They were then randomized to observation versus metronomic maintenance therapy (MMT) [first 12 weeks of daily oral celecoxib 200 mg BD, daily oral cyclophosphamide (50 mg OD) and weekly IV cisplatin (25 mg/m2)]; followed by 1 year of daily oral metformin (500 mg BD), daily oral cyclophosphamide (50 mg OD), and weekly oral methotrexate (12 mg/m2). The cost of such MMT would be Rs. 8191/- per patient [Table 5] equivalent to 109/- USD if treated in India.[17-19]
| Medication | Dose for SA 1.67 m2 | No. of doses | Price per dose in India | Price per dose in the USA | Total price in INR in India | Total price in USD in the USA |
|---|---|---|---|---|---|---|
| First 12 weeks | ||||||
| Celecoxib | 200 mg | 168 | 6.90 | 1.87 | 1159.20 | 314.16 |
| Cyclophosphamide | 50 mg | 84 | 2.67 | 0.60 | 224.28 | 50.40 |
| Cisplatin | 50 mg | 12 | 262.78 | 492.50 | 3153.36 | 5910.00 |
| Next 1 year | ||||||
| Metformin | 500 mg | 730 | 1.42 | 0.08 | 1036.60 | 58.4 |
| Cyclophosphamide | 50 mg | 365 | 2.67 | 0.60 | 974.55 | 219.00 |
| Methotrexate | 20 mg | 52 | 31.6 | 21.56 | 1643.20 | 1121.12 |
| Total Price in India versus USA | INR 8191/- (equivalent USD 109/-) | (equivalent INR 576,607/- ) USD 7673/- | ||||
Banavali et al.’s data showed that the 3-year overall survival (OS) was 100% in the MMT arm versus 54.3% in the observation arm.
If our 83 TNBC patients were all given the above MMT, 38 more patients would have survived for 3 years at the total cost of Rs. 679,853/- (Rs. 8191 per patient × 83 patients). This would translate into Rs. 5964/- per life year per patient (USD 79.6 per added year of life per patient if treated in India). If these same patients were treated in the USA, the cost would be USD 7673/- per patient and USD 636,859/- for 83 patients. This would translate into USD 5587/- per life year per patient.
HR-positive Her2-negative EBC
Of the 201 patients with EBC, 111 (55%) were HR positive as well as Her2 negative. There is a lot of interest in deciding which of these should get adjuvant chemotherapy.[20] The objective is to identify real high-risk patients and only offer CT to this subset. In addition to clinical features, the only biomarker test studied sufficiently in Indian patients is CanAssist Breast.[21,22] As per ICMR guidelines, in routine clinical practice, we should only use tests that have been validated in Indian patients.[23] This is because there is evidence of biological and genetic variations among ethnic and geographically diverse groups – a fact that has been acknowledged by 74% (n = 137/185) of participants in an online survey (personal communication Dr. Purvish M. Parikh). Among the HR-positive Her2-negative EBC patients in India, CanAssist Breast results have shown that 68% are low risk for recurrence (and should not be given chemotherapy), whereas the remaining 32% are at high risk of recurrence (and are candidates for adjuvant CT).[24] Another survey among Indian oncologists showed that the preferred chemotherapy in this setting is adriamycincyclophosphamide (AC) followed by paclitaxel[25]. The cost of such adjuvant CT would be Rs. 53,096/- per patient [Table 6] equivalent to 707/- USD.
| Medication | Dose for SA 1.67 m2 | No. of doses | Price per dose in INR in India | Price per dose in USD in the USA | Total price in INR in India | Total price in USD in the USA |
|---|---|---|---|---|---|---|
| First 4 cycles | ||||||
| Doxorubicin | 100 mg | 4 | 2170 | 58.24 | 8680 | 232.96 |
| Cyclophosphamide | 1000 mg | 4 | 154 | 231.01 | 616 | 2464.00 |
| Next 4 cycles | ||||||
| Paclitaxel | 300 mg | 4 | 10,950 | 55.70 | 43,800 | 222.80 |
| Total | INR 53,096/- (equivalent USD 707/-) | (equivalent INR 219,293/-) USD 2920/- | ||||
Among our 111 patients, this would mean that 76 patients would be spared the toxicity of adjuvant CT and 35 would benefit from it.
By avoiding overtreatment in 76 patients, the saving on the cost of chemotherapy medication itself would be INR 4,035,296/- (equivalent to USD 53,699/- if treated in India and USD if treated in the USA). In addition, there would be a substantial benefit of maintaining quality of life (QoL) by avoiding potential toxicity.
In summary, it is time to choose wisely. We discussed two important groups of breast cancer – TNBC and HR-positive Her2-negative EBC. This was possible because we now have the advantage of robust data from our own patients. Thus, it is possible to ascertain the implications of treatment options selected, discuss details with the patients, and arrive at the final treatment plan taking into consideration patient preferences.
CONCLUSION
It is possible to identify patients with low risk early breast cancer using Can assist and save them from unnecessary cost and/or toxicity.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Breast cancer statistics and prediction methodology: A systematic review and analysis. Asian Pac J Cancer Prev. 2015;16:4237-45.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiologic analysis of breast cancer incidence, prevalence, and mortality in India: Protocol for a systematic review and meta-analyses. Medicine (Baltimore). 2018;97:e13680.
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective analysis of hormone receptor profile in breast cancer patients from a tertiary cancer center in western part of India and study their relationship with her 2 Neu (IHC+FISH), age and menopausal states. Prensa Med Argent. 2018;105:1-7.
- [Google Scholar]
- American Joint Committee on Cancer Cancer Staging Manual (7th ed). New York: Springer; 2010.
- [Google Scholar]
- Available from: http://www.aboutcancer.com/AJCC_7th_breast_5.gif [Last accessed on 2020 Aug 12]
- Cost effectiveness of trastuzumab for management of breast cancer in India. JCO Glob Oncol. 2020;6:205-16.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of 1-year vs shorter durations of adjuvant trastuzumab among patients with early breast cancer: An individual participant data and trial-level meta-analysis. JAMA Netw Open. 2020;3:e2011777.
- [CrossRef] [PubMed] [Google Scholar]
- Alarming burden of triple-negative breast cancer in India. Clin Breast Cancer. 2018;18:e393-9.
- [CrossRef] [Google Scholar]
- Triple-negative breast cancer: Pattern of recurrence and survival outcomes. Indian J Med Paediatr Oncol. 2019;40:67-72.
- [CrossRef] [Google Scholar]
- Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161:279-87.
- [CrossRef] [PubMed] [Google Scholar]
- Management of primary and metastatic triple negative breast cancer: Perceptions of oncologists from India. Indian J Cancer. 2011;48:158-64.
- [CrossRef] [PubMed] [Google Scholar]
- Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer. 2018;124:2086-103.
- [CrossRef] [PubMed] [Google Scholar]
- Gene expression in triple-negative breast cancer in relation to survival. Breast Cancer Res Treat. 2018;171:199-207.
- [CrossRef] [PubMed] [Google Scholar]
- PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol Ther. 2018;19:373-80.
- [CrossRef] [PubMed] [Google Scholar]
- Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J Clin Oncol. 2018;36:543-53.
- [CrossRef] [PubMed] [Google Scholar]
- Metronomic maintenance therapy (MMT) and prevention of relapses in patients with triple-negative breast cancer (TNBC)? A retrospective analysis. J Clin Oncol. 2014;32:e12037.
- [CrossRef] [Google Scholar]
- Available from: https://www.medindia.net/drug-price [Last accessed on 2020 Aug 12]
- Available from: https://www.drugs.com/price-guide [Last accessed on 2020 Aug 12]
- Available from: https://www.xe.com/currencyconverter/convert/?Amount=1&From=INR&To=USD [Last accessed on 2020 Aug 12]
- Practical consensus recommendations on management of HR +ve early breast cancer with specific reference to genomic profiling. South Asian J Cancer. 2018;7:96-101.
- [CrossRef] [PubMed] [Google Scholar]
- CanAssist breast: An affordable breast cancer prognostic test validated on Asian patients. J Clin Oncol 202;. ;15:541.
- [CrossRef] [Google Scholar]
- Clinical validation of an immunohistochemistry-based CanAssist-breast test for distant recurrence prediction in hormone receptor-positive breast cancer patients. Cancer Med. 2019;8:1755-64.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus Document for Management of Breast Cancer. 2016. Available from: http://www.cancerindia.org.in/wpcontent/uploads/2017/11/Breast_Cancer.pdf [Last accessed on 2020 Aug 12]
- [Google Scholar]
- CanAssist breast impacting clinical treatment decisions in early-stage HR+ breast cancer patients: Indian scenario. Indian J Surg Oncol. ;2019
- [CrossRef] [Google Scholar]
- Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976-83.
- [CrossRef] [Google Scholar]






