Translate this page into:
Survey for molecular reports in practicing oncologists in India

*Corresponding author: Vikas Talreja, Department of Medical Oncology, Safdarjung Hospital, Ansari Nagar East, New Delhi - 110 029, India. vikasttalreja@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Talreja V, Parikh P, Nagar M, Kataria S. Survey for molecular reports in practicing oncologists in India. Int J Mol Immuno Oncol 2020;5(3):117-20.
Abstract
Objectives:
Molecular oncology (GO) is a discipline that focuses on the diagnosis, staging, prognosis, and management of cancer with the help of molecular genetics. Increasing understanding of the molecular changes that drive tumor progression has transformed the treatment of this disease. The main goal of this study was to describe the current situation in India regarding the knowledge, attitude, and practice of molecular oncology through an online survey of oncologists.
Material and Methods:
A descriptive survey was sent to several hospitals by means of E-mails and social media.
Results:
Between December 2019 and February 2020, 74 responses were collected. All of the respondents were interested in the accreditation of the reports and authorizing agency accrediting them. About 68.9% of the practicing oncologist did not have any provision of molecular oncology tumor board. 82.4% of the oncologists reviewed with the molecular pathologist for discussion of the molecular reports. On the contrary, 58.1% of the oncologist never received any information from the reporting team about the patient clinical details, follow-up, or changes in the reports ever. About 79.7% of the prescribing oncologist were interested in remuneration in any form for prescribing such tests. About 27% of the oncologist were not aware of any accreditation agency available in India for molecular oncology reports.
Conclusion:
From the nationwide survey, we conclude that there is an increasing perception of the need for training in molecular oncology. This survey reflects a reality, in which specific needs are perceived.
Keywords
Molecular oncology
Next generation sequencing
Molecular reports
Targeted therapy
Survey
INTRODUCTION
Molecular oncology is rapidly emerging as a promising approach in cancer treatment.[1,2] For the promise of precision oncology to be realized, oncologists will need to understand its benefits, risks, and limitations before consenting to treatment. Yet, integration of matched tumor and germline sequencing into clinical care presenting significant challenges for doctors education. Only a few studies have explored physician perspectives in the context of this precision oncology.[3,4]
Applications of molecular diagnostics to oncology have been slow to make their way to the clinical laboratory. While numerous genes and mutation spectra have been found to be involved in tumorigenesis, it is only recently that these findings begin to become useful in a clinical setting. Building on the technical knowledge obtained from molecular infectious disease testing, new instruments and assays have been developed to answer similar questions regarding qualitative, quantitative, and genotyping issues. Molecular assessment has been advocated as a way to provide detailed evaluation of the health status. Although recommended by the National Comprehensive Cancer Network and the American Society of Clinical Oncology (ASCO), molecular assessment is not routinely implemented in oncology practice as it is perceived to be time and resource consuming.[3,4] Although the time commitment and burden on patients and caregivers are concerns, recently developed cancer-specific molecular assessment tools can gather a wealth of information in a relatively short amount of time.[5,6]
To date, there is limited information on Indian oncologists’ views and experiences of molecular oncology.
This study aimed to explore the views of Indian oncologists regarding the perception of and barriers to the incorporation of molecular technology in routine clinical practice.
MATERIAL AND METHODS
This was an anonymized cross-sectional survey. The online survey, based on a literature review and expert opinion, comprised 14 questions covering [Table 1]:
|
Respondent characteristics, clinical practice environment, and patient population.
Challenges and treatment decision-making factors in the prescription, interpretation, authenticity, follow-up, and application of molecular reports.
Benefits of and barriers to the implementation of molecular oncology and tumor boards.
The questionnaire was intentionally straightforward so that it could be filled in quickly; we, therefore, did not collect respondents’ characteristics (age, gender, and type of practice). A selection bias could not be avoided, as the medical oncologists most highly motivated in molecular oncology may have been the ones who responded to the survey. We attempted to draft a simplified questionnaire that could be completed in 5–10 min, so as to capture as much participation as possible, albeit at the expense of other relevant information. The Google Forms was case sensitive; as a result, the survey could not be completed twice by the same person.
Electronic responses were automatically captured in a Google spreadsheet that was linked to the online form, and responses collected on the PDF version were manually entered into the same sheet. The survey was designed on Google Forms (Google, Mountain View, and CA).
Qualitative variables were reported as numbers (N) and percentages.
RESULTS
Among the respondents, 90.5% were interested in the molecular testing to be available in the hospital of their practice. Among those who received the reports, 86.5% wanted these to be confidential and all of them wanted to be delivered from an accredited laboratory. However, 27% of the prescribing oncologists were not aware of the agencies for the accreditation for these molecular reports. Among those who have responded, more than 77% were interested in the references and list of mentioned potential therapeutics and drug report form attached. About 50% of the prescribing oncologists were not interested in the remuneration associated in prescribing molecular tests. About 68.9% of the prescribing oncologist did not have the provision of molecular tumor board in the hospital. The factors deciding the preferences for selection of the target molecular profile are enlisted in the Figure 1 based on cost, experience, turnaround time, availability of liquid biopsy, cost, coverage, and targetable mutation. Among the molecular reports, quality of DNA, allele frequency, depth of coverage, platform performed and validation of report, and the physician choice of preferences are illustrated in Figure 2.
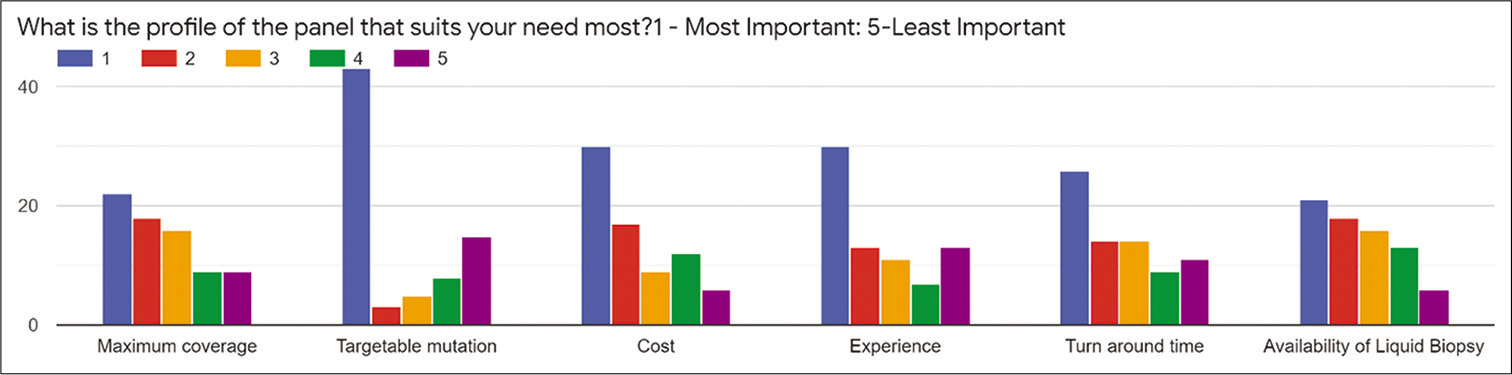
- Preferences of the oncologists for the factors affecting the choice of the panel.
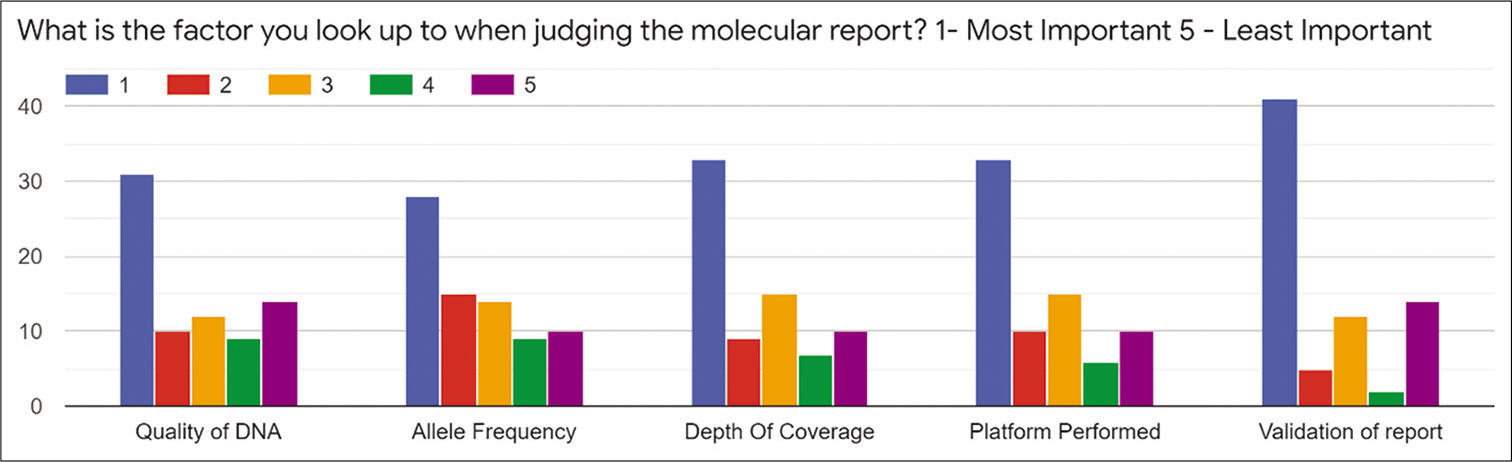
- Preferences of molecular reporting in medical oncologists.
DISCUSSION
This longitudinal survey is one of the first and largest studies to examine oncologist understanding and expectations regarding the use of next-generation sequencing in their own cancer treatment. Perhaps, our most notable finding was an apparent mismatch between physician pre-test expectations of direct benefits from cancer genome sequencing and their actual realization of such benefits. Physicians reported expecting a wide range of potential benefits from study participation, including greater understanding of underlying causes of their cancer, genomic information of relevance to their family members’ health, and the ability to enroll more of their patients in the revolution of molecular oncology. Although our study highlighted areas for improvement in oncologists education, it should be noted that participants in the study showed good knowledge of many basic facts about molecular oncology and sequencing.
1. Our findings should be interpreted in light of several study limitations. The study sample of oncologists was disproportionate to those who are prescribing in real-life limiting generalizability of study results. Future research in this area should attempt to enroll oncologists with greater diversity in terms of geography, institution of practice (academic/private), and accessibility to molecular laboratory. Another study limitation was that some key measures had either not been formally validated (e.g., international guidelines or policies) or were not administered in their entirety (e.g., decisional regret), which may have introduced some measurement error into the assessment of key outcomes. The authors agree that tissue availability is a valid concern, but note that there have been significant advances in tumor DNA extraction from low-purity samples and with the advent of blood- based NGS testing. These allow for either the extraction of tumor DNA from as little as 5% tumor purity samples or from the isolation of circulating cell-free DNA if tissue biopsies are not available. While blood-based NGS assays have their distinct limitations, there remains a significant benefit to analyze circulating tumor DNA for routine clinical care of cancer patients. Hand in hand with the issue of tissue scarcity is the perception that NGS-based tumor testing rarely identifies mutations with clinical significance or improves clinical outcomes. The most widespread argument against NGS testing is the additional cost to both patients and payers with little clinical benefit to justify the cost. Recent FDA approvals and Centers for Medicare and Medicaid Services (CMS) coverage guidelines demonstrate the emerging clinical utility of NGS-based tests. A 2017 review of both clinical and cost-effectiveness of NGS testing concluded that NGS-based testing is effective in identifying actionable mutations in cancer and aided in matching 37% of the patients to targeted therapies. Access to molecular tumor boards, limited discussion of supplementary reports, discussion on variance of unknown origin, and adequacy of material were reported as other barriers.
In addition, some commentators have noted that surveys such as ours that demonstrate participant desires for hope that research participation will improve their own personal care while recognizing that such clinical benefits are not the primary purpose of a given study.
Limitations
Despite the low survey response rate, the findings are consistent with surveys conducted in other countries. Respondents likely had limited direct exposure to molecular oncology services given the small number of services in India.
Conversely, it is likely that there is a degree of responder bias, with those with an interest in molecular oncology likely overrepresented in the sample.
Implications for future practice/service design
This study has identified a desire and perceived need for increased molecular oncology services and collaboration with nationwide molecular oncologists among respondents. The design and provision of molecular oncology services must address barriers identified in this research, in particular the availability and molecular expertise in a timely manner. Closer collaboration between oncologists and molecular expertise is required to embed such services into routine practice. However, the low response rate may also reflect an ambivalence toward molecular oncology among oncologists, highlighting the need for ongoing awareness raising and generation of supporting evidence. Incorporation of molecular genetics into oncology training, and vice versa, may build expertise into the future workforce, and should be considered by the respective training organizations. While it will not be feasible for all adults with cancer to see a dual-trained molecular oncologist, these highly skilled specialists are ideally placed to guide future research and education in the field.
The preferred model of care may be best addressed locally, tailored to the local availability of molecular expertise and funding models. Ongoing research is required to build evidence regarding the merits of differing models of care and the benefits of molecular driven interventions in this population.
CONCLUSION
These challenges, together with perceived funding shortfalls, should inspire educational, training, and other interventions to ensure that developments in molecular oncology can result in optimal cancer care. To disseminate the use of a molecular assessment and the various available screening tools, education of oncologists and molecular oncologists tools that are validated, efficient, and predictive of outcomes is needed. In short, molecular oncology in our country has only just begun – humbly, yet unstoppably.
ACKNOWLEDGMENTS
We want to thank all the members that have participated in the interview.
Research involving human participants and/or animals
The work has been conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Purvish Parikh is the editor of this journal. He has no conflict of Interests.
References
- Personalized medicine and development of targeted therapies: the upcoming challenge for diagnostic molecular pathology. Virchows Arch. 2006;448:744-55.
- [CrossRef] [PubMed] [Google Scholar]
- Genomics and proteomics: Emerging technologies in clinical cancer research. Crit Rev Oncol Hematol. 2007;61:1-25.
- [CrossRef] [PubMed] [Google Scholar]
- Obtaining informed consent for clinical tumor and germline exome sequencing of newly diagnosed childhood cancer patients. Genome Med. 2014;6:69.
- [CrossRef] [PubMed] [Google Scholar]
- Patient/parent perspectives on genomic tumor profiling of pediatric solid tumors: The individualized cancer therapy (iCat) experience. Pediatr Blood Cancer. 2016;63:1974-82.
- [CrossRef] [PubMed] [Google Scholar]
- NCCN guidelines insights: Older adult oncology, version 2.2019. J Natl Compr Canc Netw. 2019;14:1357-70.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi160-70.
- [CrossRef] [PubMed] [Google Scholar]






