Translate this page into:
Outcomes with atezolizumab and bevacizumab in hepatocellular carcinoma - Real-world data from India

*Corresponding author: Nikhil Sebastian, Department of Medical Oncology, Apollo Cancer Centre, Chennai, Tamil Nadu, India. drnikseban@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sebastian N, Nawaz S, Ananthakrishnan R, Kumanan J, Chandran C, Raja T. Outcomes with atezolizumab and bevacizumab in hepatocellular carcinoma - Real-world data from India. Int J Mol Immuno Oncol 2023;8:102-5.
Abstract
Objective:
The current standard of treatment for advanced hepatocellular carcinoma (HCC) is a combination of immunotherapy and anti-vascular endothelial factor antibody therapy or dual agent immunotherapy. To describe the real world outcomes of atezolizumab and bevacizumab therapy in advanced HCC.
Materials and Methods:
Retrospective data of patients treated with this regimen in our institution from April 2020 to March 2023 was accessed from electronic records and data of 10 patients were analyzed.
Results:
The median duration of follow up is 18.40 (8.40 to 20.26) months. The median progression free survival was 13.16 (7.53 to 18.76) months. The median overall survival was 18.40 (8.40 to 20.26) months.
Conclusion:
The combination of Atezolizumab and Bevacizumab is a safe and feasible option for the treatment of advanced HCC.
Keywords
Hepatocellular carcinoma
Immunotherapy
Atezolizumab
Bevacizumab
Overall survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is a moderately chemoresistant tumor and the outcome with cytotoxic chemotherapy in advanced HCC has been dismal in the pre-tyrosine kinase inhibitor (TKI) era. From 2008 onwards, the anti-vascular endothelial growth factor (VEGF) TKI sorafenib proved to be of benefit,[1] followed by other small molecule TKIs like lenvatinib[2] as first-line therapies and regorafenib as second line on progression with sorafenib.[3]
There was no agent proven to be superior to sorafenib in advanced HCC until the era of immunotherapy started when bevacizumab and atezolizumab combination therapy showed benefit.[4] This combination improved survival as compared with first-line TKI in advanced HCC in phase III trial.[5] At present, atezolizumab plus bevacizumab is indicated for those patients with Child-Pugh Class A cirrhosis, an excellent performance status, and those who have no contraindications to bevacizumab. There are limited publications about the outcomes of this combination in real-world settings and also in settings outside the trial criteria. We present a case series and follow-up of ten patients who have received atezolizumab plus bevacizumab for advanced HCC in an Indian tertiary cancer center.
MATERIALS AND METHODS
Retrospective data of all patients diagnosed with advanced HCC and received systemic therapy with atezolizumab and bevacizumab as first- or second-line therapy in our institution, Apollo Cancer Center, Chennai, from April 2020 to March 2023, were obtained from the electronic medical records. Atezolizumab-bevacizumab protocol was administered as 1200 mg of atezolizumab plus 15 mg/kg of body weight of bevacizumab intravenously every 3 weeks and continued until disease progression or unacceptable toxicity. The statistical analysis of the data was done using SPSS version 26, and the demographic data were summarized using descriptive statistics. The analyses of median progression-free survival (PFS) and overall survival (OS) were performed with the use of a stratified log-rank test and were expressed as their medians with interquartile ranges.
RESULTS
There were a total of ten patients with advanced HCC who received atezolizumab and bevacizumab, among which seven were male. The mean age was 64.5 ± 8.0 years. Four of them had histological evidence with three of them proven with a core biopsy and one with needle cytology. The rest of the patients were diagnosed based on the radiological features and Alpha-Fetoprotein (AFP). The AFP at diagnosis was 747.95 (interquartile range 111.25–6116.25) units per liter. Three of them had prior chronic liver disease, but none were diagnosed to have hepatitis B or C. The mean serum bilirubin levels were 1.4 ± 1.93 mg/dL, and three of the patients had raised serum bilirubin. The mean albumin levels were 3.67 ± 0.49 mg/dL, with five of them having low albumin levels. Two patients had mild to moderate ascites and none of them had encephalopathy or any abnormalities in coagulation parameters.
The mean number of lesions in the liver was 2.3, and five patients had lesions in both lobes of the liver. Three of them had portal vein thrombus and all three were tumor-containing thrombi.
Three patients had barcelona clinic liver cancer (BCLC) stage A and seven had advanced disease with stage C. There were distant metastatic lesions in five of the patients. The Child-Pugh score was A for seven patients and B for three patients. Out of these three patients, two had a score of 7, and one had a score of 8.
Five patients had systemic hypertension, one of them had coronary artery disease (had undergone coronary artery bypass graft surgery in the past), five had diabetes mellitus, and two of them had hypothyroidism as comorbidities. All together six patients were on medications for their comorbid conditions. One of them had bone metastases and was on denosumab.
The treatment was the first line of systemic therapy for nine out of ten patients. One patient had received two prior lines with TKIs-Lenvatinib for 12 months and on progression, cabozantinib for 3 months.
Among the nine patients who received AB as the first systemic therapy, one had undergone complete excision of the liver lesion with negative margins, and the other had received a TACE procedure.
Two patients had missed doses during the course of the therapy. One of them had missed a single dose of both atezolizumab and bevacizumab. The other patient had hemiplegia with imaging showing small-vessel ischemic changes, due to which further doses of the bevacizumab were withheld after the 11th dose, but continued the atezolizumab alone till the 18th dose until disease recurrence.
The overall response rate was 50% with five out of ten patients having partial responses. It is worthwhile noting that the five patients who had no partial response at first assessment (stable disease) had progression during subsequent follow-ups. Four patients died, of which three were the ones that did not have a partial response in any of the assessments, and one patient with PR died due to cardiac arrest [Figure 1].
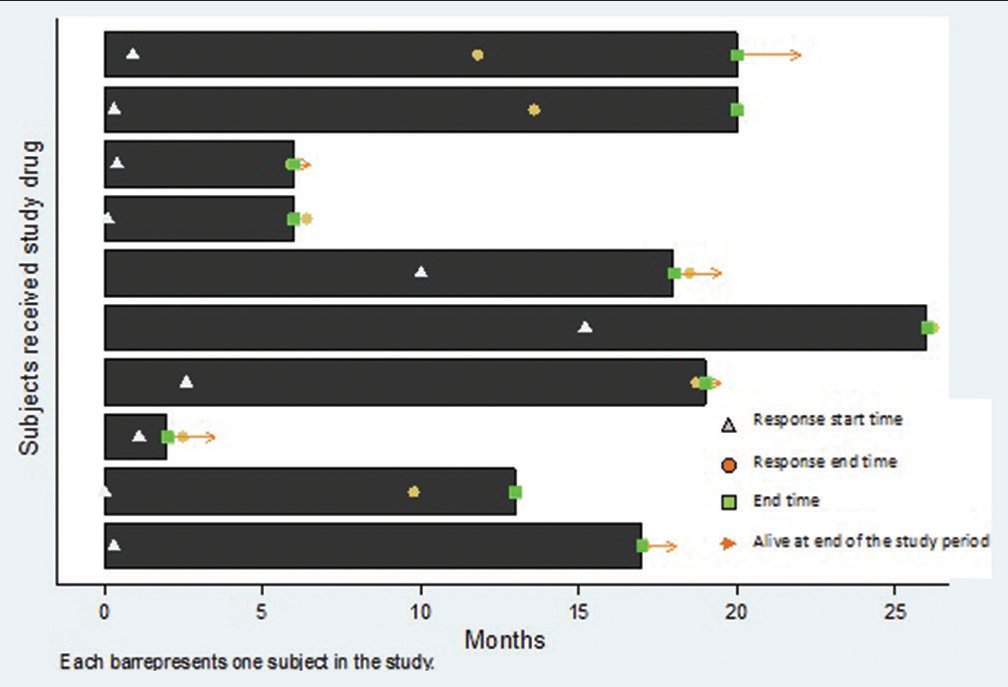
- Swimmers plot depicting the events of the start of response, progression, and death during the course of follow-up. The triangle depicts the start of response, the circle shows the end of response (progression), the square represents the time point of last assessment. The bars with arrows at the end represent those who are still alive.
The median duration of follow-up is 18.40 (8.40–20.26) months. The median PFS was 13.16 (7.53–18.76) months. The median OS was 18.40 (8.40–20.26) months.
Four of the patients who were <60 years old had a follow-up of 18.5 months (13.9–19.6) with a PFS of 14.96 (9.8–18.13) months and OS of 18.5 (13.9–19.6) months. The rest of the six patients with an age more than 60 years with a median follow-up of 16.36 (10.13–19.96) months had a median PFS of 12.13 (8.4–18.13) months and median OS of 16.36 (8.4–18.13) months. The PFS and OS were numerically lower for those with an age of more than 60 years, but this difference was not statistically significant.
Median PFS and OS were not significantly different between different BCLC stages. The median PFS and OS for the three patients with BCLC stage C were 10.17 months and 13.63 months, respectively, while both median PFS (P = 0.518) and OS (P = 0.187) were 19.1 months and 19.36 months, respectively, for the rest of the patients with BCLC stage A. There was no statistically significant difference between the PFS and OS between the different Child-Pugh grades and albumin bilirubin ratio (ALBI) scores.
DISCUSSION
The initial evidence for the feasibility of combination immunotherapy and anti-VEGF therapy with atezolizumab and bevacizumab came from the phase Ib GO-30140 trial.[4] The study included five cohorts, and the two cohorts – Groups A and F comprised patients with HCC. In Group A, all patients received atezolizumab (1200 mg) and bevacizumab (15 mg/kg) intravenously every 3 weeks. In Group F, patients were randomly assigned (1:1) to receive the same drug regimen as group A every 3 weeks or atezolizumab alone. There was an objective response rate of 35% in group A. In Group F, a comparison between single-agent atezolizumab and the combination showed superior PFS for the combination arm (5.6 months vs. 3.4 months). The efficacy of the combination was further proved in the IMBrave-150 trial.[5]
Several studies have reported the outcome of the combination in real-world settings[6-10] including one study from India.[11]
The median PFS of our study patients (13 months) is higher compared to most studies, where it ranges from 5.1 months to 8 months. The OS also shows a higher trend with 18 months in this study with OS in other studies ranging from 11.7 months to 15.7 months.[6-9,11] This may be due to the predominantly prior treatment-naive patients in this study.
About 70% of patients in our study were newly diagnosed with advanced HCC with no other prior local or systemic therapies, and only one had undergone a prior line of TKI therapy. About 60%–70% of patients had undergone prior treatments in studies by D’Alessio et al.[6] and De Castro et al.[9]
None of the ten patients were positive for hepatitis viral infections in this study, while this fraction was 33–65% in various other studies.[4,6,8,9,11] The percentage of patients with Child-Pugh B status (around 30–40%),[6,8,9,11] BCLC-C stage (70–80%),[6,8,9,11] and portal vein thrombus (around 40%)[6,8] was comparable with that of other studies.
The higher PFS and OS seen in our study may be due to the higher proportion of treatment-naive patients and the smaller sample size.
CONCLUSION
The combination of atezolizumab and bevacizumab is a feasible option for treating advanced HCC in real-world settings.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-90.
- [CrossRef] [PubMed] [Google Scholar]
- Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-73.
- [CrossRef] [PubMed] [Google Scholar]
- Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
- [CrossRef] [PubMed] [Google Scholar]
- Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808-20.
- [CrossRef] [PubMed] [Google Scholar]
- Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894-905.
- [CrossRef] [PubMed] [Google Scholar]
- Real-world use of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-pugh A and B cirrhosis. J Clin Oncol. 2022;40(4 Suppl):393.
- [CrossRef] [Google Scholar]
- Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-pugh A and B cirrhosis: A real-world study. Hepatology. 2022;76:1000-12.
- [CrossRef] [PubMed] [Google Scholar]
- Reproducible safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice: Results of the AB-real study. Eur J Cancer. 2022;175:204-13.
- [CrossRef] [PubMed] [Google Scholar]
- Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: A real-world experience. Ther Adv Med Oncol. 2022;14:17588359221080298.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int. 2022;42:674-81.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of atezolizumab-bevacizumab in real world: The first Indian experience. J Clin Exp Hepatol. 2023;13:618-23.
- [CrossRef] [PubMed] [Google Scholar]







