Translate this page into:
Nanobytes: Innovative approaches in molecular medicine

*Corresponding author: Radhika A. Vaishnav, Department of Molecular Informatics, Natureka Life Sciences, Vadodara, Gujarat, India. radhikavaishnav@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vaishnav RA, Mehta DG. Nanobytes: Innovative approaches in molecular medicine. Int J Mol Immuno Oncol 2021;6(1):4-5.
ERYTHROCYTE-ANCHORED SYSTEMIC IMMUNOTHERAPY
Cancer progeny is the true 007 of the cancer saga. The metastasis only happens after the tumor crosses a Rubicon in new and further gene change. The metastatic cells (mets) are the most adroit in travel and intrepid in survival as they have undergone changes. To quote Robert Weinberg: “end-products of a multi-step cell-biological process termed the invasion-metastasis cascade, which involves dissemination of cancer cells to anatomically distant organ sites and their subsequent adaptation to foreign tissue microenvironments.”[1]
Profuse capillaries in close interplay make lungs a rich target for mets. They get trapped there, hibernate, and create immune sanctuaries. “Induction of an immune response at the metastatic site is also hampered by the rapid development of an immunosuppressive microenvironment.”[2]
Systemically given drugs must clear the liver, and to be effective, have to be given at a higher dose to compensate. This is where using red blood cells as a conduit to carry chemokine CXCL10 encapsulated in a nanoparticle and delivered to lungs creates a therapy option. Such a technique was recently reported in mice which used erythrocyte-anchored systemic immunotherapy (EASI) that would control progression of mets and generate an adaptive response for tumor suppression.[2]
“We are very excited about this work” says Dr. Samir Mitragotri, Hiller Professor of Bioengineering and Hansjorg Wyss Professor of Biologically Inspired Engineering at Harvard University. “It offers a new way of approaching cancer, especially after its metastasis. Currently, very few options exist for treating lung metastasis. Often, metastasis has a poor prognosis. We wanted to exploit the unique opportunity provided by lung metastasis to treat cancer. Typically, cancer cells are well protected within the tumors due to the stroma and immunosuppressive environment. However, when the cancer cells leave the tumor and metastasize in the lungs, they are much more exposed. Taking advantage of this exposure, we sought to target them using our unique delivery mechanism. RBC-hitchhiking nanoparticles target lungs with high efficiency, thus depositing large quantities of chemokines in the lungs so as to establish the necessary gradient for the infiltration of immune cells to locally kill cancer cells as well as raise a systemic immune response.”
In case of lung metastasis, local chemokines have been depleted and an environment is created which does not attract white blood cells, thus protecting the tumor cells. The simplicity of design of EASI involves the engineering of one’s own red blood cells to achieve the targeted delivery of immune-activating molecules. The targeting in the case of the above-mentioned mouse experiment was performed using the adhesion molecule ICAM-1. Once the red blood cells reached the lungs they would release CXCL10. The researchers noted better survival, fewer metastatic nodules, and presence of CD8 memory cells that could minimize recurrence in the EASI treated mice as compared to controls.
So imagine the EASI as the “Robin Hood,” well-disguised as normal red blood cells, that seek out the mets that are hiding behind an armory of blood vessels. Once at the target site, the EASI releases chemokine signals to recruit more “bandits” – the natural immune arsenal of the body as illustrated in Figure 1.
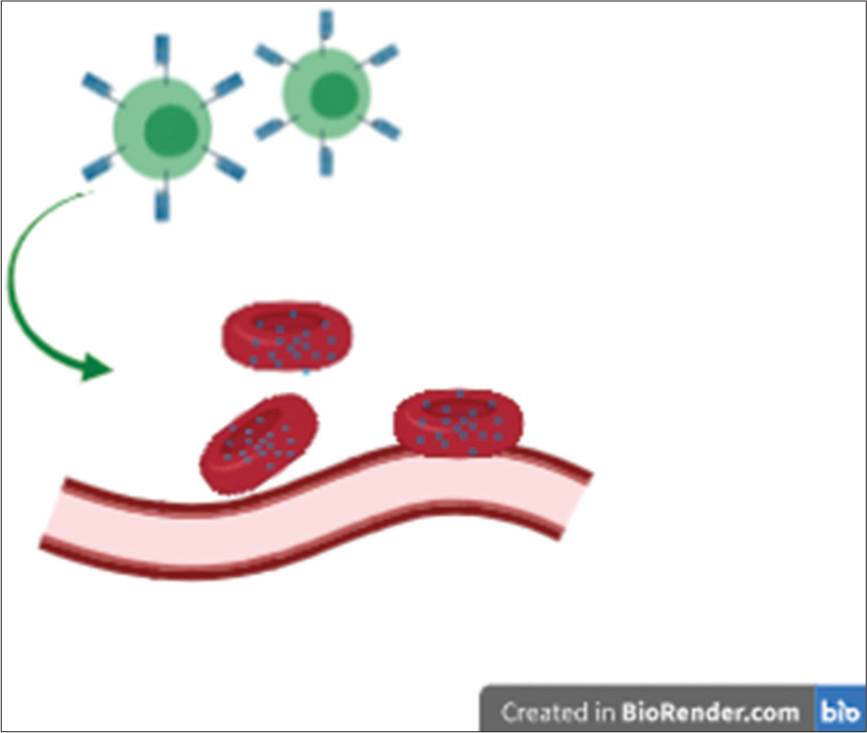
- Erythrocyte anchored systemic immunotherapy uses chemoattractant molecules to recruit immune cells via a piggy-back approach on natural red blood cells.
Truly, the engineer-biologist synergy and transdisciplinary research can lead to ingenious advances in cancer therapy.
RNA VACCINES – A PARADIGM SHIFT
The smallpox vaccine in 1796 was a serendipitous discovery by Edward Jenner who performed arm-to-arm inoculation of substance taken from a cowpox blister. In the first half of the 20th century, pertussis, diphtheria, AND tetanus (DTP) entered the vaccination scene. The polio scare in the mid-1900s took a toll as people waited for a vaccine before they could resume normalcy. In 1955, the development of the polio vaccine by Jonas Salk brought much awaited relief, although eradication is yet to be realized worldwide. In the 1960s, measles, mumps, and rubella became preventable thanks to the vaccines that formed the MMR combination in 1971. Then in the 1970s, smallpox was declared as completely eliminated. A historical milestone set the pace for next stage in development which took off in the following decades leading to introduction of new vaccines such as hepatitis B, influenza or varicella, as well as improvement in the existing ones.[3]
A paradigm shift occurs infrequently, and in a well-oiled machine that vaccines seem to be, one would not expect many major changes. The 2020 pandemic accelerated work on all fronts of vaccine development and new modes of immunization came to light. RNA vaccines were already under clinical trials for various cancers. The ability to deliver targeted nanoparticles allowed for the design of RNA that would encode the target antigen only after entering cells in the body. RNA itself is non-inheritable and cannot be integrated into the human genome. This makes it safer than a DNA vaccine in terms of long-term consequences. mRNAs are not as stable as DNA, though, and require special storage conditions such as low temperatures or other methods of improving shelf life.[4] The spectrum of cancers for which RNA vaccine clinical trials are either ongoing or completed includes AML, CML, mesothelioma, glioblastoma, renal cell carcinoma, pancreatic cancer, melanoma, non-small cell lung cancer, prostate cancer, ovarian cancer, multiple myeloma, and colorectal cancer.[5]
As diseases continue to be eradicated, in the future, one hopes cancer will join the list.
References
- Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275-92.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nat Biomed Eng 2020
- [CrossRef] [Google Scholar]
- Vaccine History Children's Hospital of Philadelphia. 2020. Available from: https://www.chop.edu. [Last accessed on 2020 Jun 12]
- [Google Scholar]
- Nanomedicine and the COVID-19 vaccines. Nat Nanotechnol. 2020;15:963.
- [CrossRef] [PubMed] [Google Scholar]
- mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-79.
- [CrossRef] [PubMed] [Google Scholar]





