Translate this page into:
Metastatic endometrial carcinoma responsive to pembrolizumab

*Corresponding author: Goutham Gandham, Department of Medical Oncology, Amrita Institute of Medical Sciences and Research Centre, Amrita Vishwa Vidyapeetham, Kochi - 682041, Kerala, India. gouthamkdd@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gandham G, Jayamohanan H, Rajanbabu A, Pavithran K. Metastatic endometrial carcinoma responsive to pembrolizumab. Int J Mol Immuno Oncol 2021;6(1):50-3.
Abstract
Carcinoma of the endometrium is the second most common and the fourth leading cause of mortality due to gynecological cancer among women worldwide. About 80% of endometrial carcinomas present as localized disease and have a 5-year survival rate of more than 95%. Most of the recurrent and metastatic endometrial cancers have a poor prognosis, and the response to chemotherapy is poor. The treatment options for advanced and recurrent endometrial carcinoma are limited. Several trials investigated the role of immune checkpoint inhibitors in endometrial cancer. Based on these trials, pembrolizumab was approved for individuals with unresectable recurrent or metastatic disease. In the current era of advancing immunotherapy, identification of mismatch repair deficiency or microsatellite instability status can predict response to drugs like pembrolizumab. Here, we report a 62-year-old lady with metastatic endometrial cancer who progressed on first-line therapy with lung and lymph nodal metastases. She was oxygen dependent and was bedridden. As she was not fit for chemotherapy, and her MSI status was found to be unstable, she was treated with pembrolizumab and had a remarkable recovery.
Keywords
Mismatch repair deficiency
Programmed cell death protein 1
Pembrolizumab
Microsatellite instability-high
Endometrial carcinoma
INTRODUCTION
Carcinoma of the endometrium was the second most common and the fourth leading cause of mortality due to gynecological cancer among women worldwide.[1] While the majority of patients present with a localized disease with an excellent prognosis, subsets had metastatic disease at presentation or develop distant recurrence after initial treatment of the primary. Mismatch repair (MMR) status is important in assigning the optimal second-line therapy in this population. The US Food and Drug Administration has approved the immune checkpoint inhibitor (ICI), pembrolizumab for the treatment of MMRd or MSI cancers, including endometrial cancer that has progressed in the prior treatment for which there are no satisfactory alternative treatment options. Here, we report a case of metastatic endometrial cancer who had a remarkable response to pembrolizumab.
CASE REPORT
A 62-year-old postmenopausal lady presented with abnormal uterine bleeding. An ultrasonogram of abdomen and pelvis was done, which showed a thickened endometrium of 25 mm. She underwent hysteroscopy with dilatation and curettage. Histopathology of endometrial curetting was suggestive of high-grade endometrioid carcinoma. Her ECOG performance status was 0, and on examination, a hard supraclavicular lymph node of 2 × 1.5 cm was felt on the left side. Per speculum examination showed hypertrophied cervix with a hyperemic lesion on the anterior lip. Pervaginal examination showed retroverted bulky uterus, pouch of Douglas nodularity, with normal parametrium and adnexae. MRI pelvis showed irregular polypoidal hypoenhancing lesions along the endometrial lining involving more than half of myometrial thickness, predominantly the anterior myometrium without extension beyond uterine serosa and with significant para-aortic lymphadenopathy. FNAC from the left supraclavicular lymph node was suggestive of metastatic adenocarcinoma. Considering the metastatic disease, she was started on chemotherapy with paclitaxel and carboplatin. Reassessment whole-body PET CT after three cycles of chemotherapy showed disease progression with multiple nodal and pulmonary metastases. The case was discussed in the multidisciplinary tumor board and was planned for palliative second-line chemotherapy.
Meanwhile, she was admitted in ICU with massive pericardial effusion and features suggestive of cardiac tamponade. Pericardiocentesis was done, and cytology was negative for malignant cells. During her hospital stay, she developed cardiac arrest and was revived after cardiopulmonary resuscitation. She was gradually weaned off the ventilator and discharged on domiciliary oxygen support. Chemotherapy was deferred because of her poor performance status. She had multiple admissions for worsening dyspnea. The pulmonary angiogram showed segmental pulmonary arterial thrombi and progression of lung metastases. She was started on anticoagulants, and microsatellite instability analysis by PCR (MSI PCR) from formalin-fixed paraffin-embedded tissue showed microsatellite instability (MSI-high [MSI-H]). The patient continued to be on 2 L/min oxygen support. Because of MSI-H tumor, the possible benefits and risks of immunotherapy were discussed with the family. The family agreed and was started on immunotherapy with injection pembrolizumab 200 mg once every 3 weeks. After three courses of pembrolizumab, she was off oxygen, and her general condition improved and was able to walk with support. Reassessment whole-body PET CT after three courses showed a stable size of primary endometrial cavity lesion with an increase in the extent of FDG uptake. However, there was a significant reduction in size and FDG uptake of lymph nodal and lung metastases [Figure 1]. Post six courses of inj. pembrolizumab 200 mg once every 3 weeks, her general condition improved, and she was able to do her daily routine activities. As of now, she received a total of nine doses of pembrolizumab 200 mg once every 3 weeks. On every follow-up, she was screened for immunotherapy side effects with liver function tests, blood sugar level, and thyroid function tests which were normal. She tolerated pembrolizumab well and never complained of skin rash or loose stools. She was planned to continue pembrolizumab until disease progression or occurrence of any unacceptable toxicities. As the size of the primary lesion was remaining stable, and the sites of metastasis had responded well, it was decided to give consolidative radiotherapy to the primary lesion.
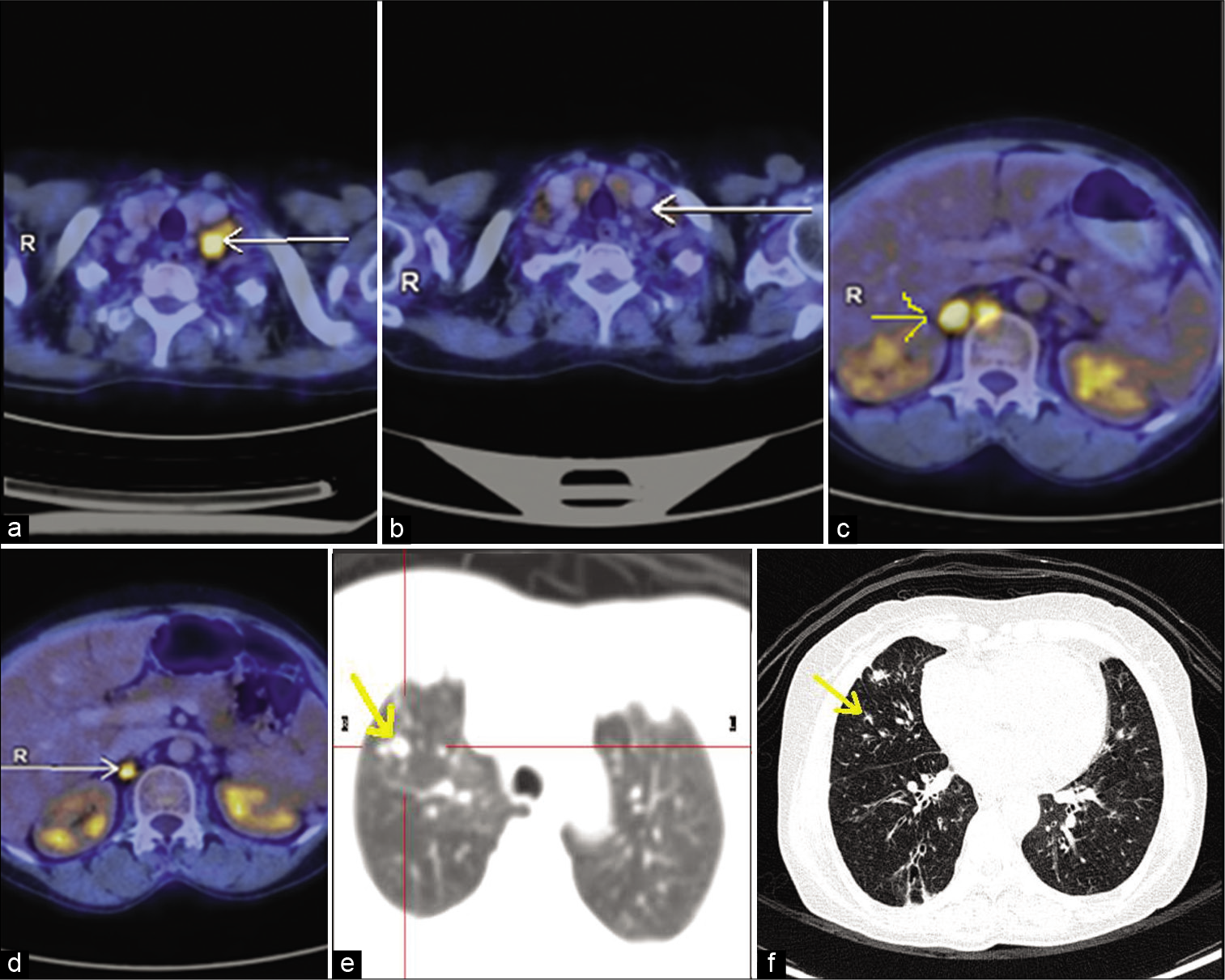
- (a) PET scan image of metastatic left supraclavicular lymph node before starting immunotherapy (arrow shows FDG avid cervical lymph node). (b) Metabolic resolution with reduction in size of metastatic left supraclavicular lymph node on response evaluation PET scan after three cycles of pembrolizumab (arrow shows cervical lymph node complete metabolic resolution). (c) PET scan image of metastatic para-aortic, aortocaval, and retrocrural lymph nodes before starting immunotherapy (arrow shows FDG avid para-aortic lymph node). (d) Metabolic resolution with reduction in size of metastatic para-aortic, aortocaval, and retrocrural lymph nodes on response evaluation PET scan after three cycles of pembrolizumab (arrow shows reduced size and FDG avidity). (e) PET scan images of metastatic nodules scattered in both lungs before starting immunotherapy (arrow shows non-FDG-avid lung nodule). (f) Reduction in size and number of fdg non-avid subcentimetric nodules scattered in both lungs on response evaluation PET scan after three cycles of pembrolizumab (arrow shows reduced size of non-FDG-avid lung nodule).
DISCUSSION
Most of the women with metastatic endometrial carcinoma would have been previously treated for localized disease and eventually develop distant relapse or disease progression. As recurrent or metastatic endometrial carcinoma can be treated with immunotherapy, MMR status is done on primary tumor or on biopsy obtained at the time of metastatic disease. ICIs have shown efficacy in many advanced solid tumors, with MMRd or MSI cancers.[2] Pembrolizumab, an IgG4 isotype antibody, has the potential to bind and block PD-1 receptors.[3] It is used for cancers that are characterized by MSI or MMR deficiency. The treatment for cancer with these characteristics is proved to be particularly effective due to their augmented somatic hypermutation. The correlation between the expression of PD-1 and PD-L1 with MMR status was appraised in various studies on solid tumors, including endometrial cancers.[4]
Treatment with pembrolizumab is usually continued till progression or unacceptable side effects. Immunotherapy is usually tolerated but is associated with toxicities including, gastrointestinal, dermatologic, hepatic, endocrine, and other less common inflammatory events. In a Phase II study of pembrolizumab including nine patients with progressive non-colorectal MMRd metastatic cancers, these patients experienced an objective response rate of 71% and a 20-week immune-related PFS rate of 67%.[5] Similarly, in a separate Phase II trial including 49 patients with MSI-H/dMMR, treatment refractory endometrial cancer, the objective response rate was 57%, and median PFS was 26 months.[6] In another study which included 15 patients with endometrial cancer with tumor mutation burden ≥10 mutations/megabase, pembrolizumab was associated with a response rate of 47%.[7] In a Phase IB study (KEYNOTE-028), out of 24 cases with locally advanced or metastatic, pretreated endometrial cancer with at least 1% of tumor cells staining for PD-L1, 13% achieved a partial response, 13% had stable disease, with a median duration of 25 weeks. Grade 3 treatment-related toxicities occurred in 17%.[8] FDA has approved the combination of pembrolizumab plus lenvatinib for its use in advanced endometrial carcinoma that is not MSI-H or dMMR, is progressive on prior systemic therapy, and that cannot be controlled using curative surgery or radiation.
In KEYNOTE-146/study 111 (a single-arm, open-label trial) involving 94 patients with MSI-H or dMMR metastatic endometrial cancers who had progressed on one prior systemic therapy, the objective response rate to pembrolizumab with lenvatinib was 36%, with a complete response rate of 2%.[9] For those responding, the probability of having a response duration of ≥6 months was 85%. The abscopal effect is mediated by a systemic anti-tumor immune response and reflects the regression of nonirradiated metastatic lesions at a distance from the primary site of irradiation. Combination of both immunotherapy and radiotherapy may help in achieving the abscopal effect, which can result in an augmented response in the irradiated site as well as the metastatic regions away from the site of irradiation.[10] Further research is warranted in endometrial carcinoma to validate newer immunotherapy agents, to find new combination therapy of immunotherapy with chemotherapy, radiotherapy for advanced endometrial cancer and to discover new biomarkers that could accurately predict the response to immunotherapy.
CONCLUSION
Recent advancements in cancer immunotherapy have resulted in the increasing use of immune checkpoints inhibitors in the treatment of refractory cancers. ICIs are beneficial in advanced cancers with MSI-H/MMRd status; hence, it would be advantageous to assess the MMR status at the time of diagnosis itself.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [PubMed] [Google Scholar]
- Association of polymerase E-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319-23.
- [CrossRef] [PubMed] [Google Scholar]
- The role of PD-1 checkpoint inhibition in gynecologic malignancies. Curr Treat Options Oncol. 2018;19:70.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598-608.
- [CrossRef] [PubMed] [Google Scholar]
- PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-20.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37:1470-8.
- [CrossRef] [PubMed] [Google Scholar]
- United States Prescribing Information. 2020. US National Library of Medicine. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s071s090lbl.pdf [Last accessed on 2020 Jun 18]
- [Google Scholar]
- Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: Results from the KEYNOTE-028 study. J Clin Oncol. 2017;35:2535-41.
- [CrossRef] [PubMed] [Google Scholar]
- Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 2020:JCO1902627. [Published online ahead of print on 2020 Mar 13]
- [CrossRef] [PubMed] [Google Scholar]
- Abscopal effect, from myth to reality: From radiation oncologists' perspective. Cureus. 2019;11:e3860.
- [CrossRef] [Google Scholar]






