Translate this page into:
Spectrum of lymphomas in India

*Corresponding author: Susmita Sarma, Department of Pathology, Center for Oncopathology, Mumbai, Maharashtra, India. susmita.sarma@accf.in
-
Received: ,
Accepted: ,
How to cite this article: Sarma S, Mehta J. Spectrum of lymphomas in India. Int J Mol Immuno Oncol. 2024;9:16-24. doi: 10.25259/IJMIO_18_2023
Abstract
Objectives:
Universal variability in the distribution of lymphomas has been reported for ages, and analyzing a large number of lymphoma cases is essential for proper insight into the disease. This study aims to obtain relative frequencies of lymphomas in India and compare them with different regions within the country, as well as with the rest of the world.
Material and Methods:
In our study, lymphoma data from two years (2019–2021) were studied, and based on morphology and immunohistochemistry (IHC), the lymphomas were subclassified according to the World Health Organization classification prevalent at the time.
Results:
The present study consisted of 2505 cases. Hodgkin lymphoma (HL) constituted 22.5%, while non-Hodgkin lymphomas (NHLs) constituted 77.5%. B-cell NHLs accounted for 85%, and T/NK cell neoplasms accounted for 15% of the NHL cases. The top two subtypes of NHL were diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma. DLBCL-not otherwise specified was further subcategorized into the germinal center type and activated B-cell type by Hans algorithm, and cases with double-expressor phenotype were also documented.
Conclusion:
The frequency of some of these neoplasms was similar, whereas some showed variations when compared to other Indian, Asian, and Western literature. The high frequency of a few T/NK cell lymphomas, DLBCL and its subtypes and some rare B/T-NHLs are the salient features of this study.
Keywords
Histological subtypes of lymphoma
Immunohistochemistry
Prevalence of lymphoma in India
World Health Organization classification
INTRODUCTION
The World Health Organization (WHO) classification of lymphoma is still evolving, and it comprises different subtypes of Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL), which are fairly heterogeneous with regard to their morphological, molecular, and clinical course.[1,2]
About 90–95% of lymphomas arise from neoplastic transformation of B-cells, whereas the rest originate from either T- or NK-cells.[3] The distribution of NHL varies across different geographic regions[4], with the most common NHL being diffuse large B-cell lymphoma (DLBCL), constituting one-third of all cases.[5]
The first discovered lymphoma was HL, which is also a B-cell neoplasm and is classified based on the phenotype of the lesional large cells into classical HL (CHL) and nodular lymphocyte predominant HL (NLPHL).[3,6,7]
This study was done to classify lymphomas according to the WHO classification prevalent at the time and to analyze the distribution of various subtypes of these neoplasms in a single center. With this context, we reviewed a total of 2505 lymphoma cases, with the aim to obtain relative frequencies of lymphomas in India and compare them with different regions within the country, as well as with the rest of the world.
MATERIAL AND METHODS
Retrospective analysis of 2505 cases was done from September 2019 to August 2021 in a referral center in Mumbai.
Due to the large number of cases received as consult cases at our referral center, these cases represent a spectrum from across the length and breadth of the country and, hence, can be considered as representing cases from all over the country. Relevant clinical data were obtained from the computer database. All cases were reviewed by two pathologists (JM and AB). In case of difficult histopathological diagnosis, we resorted to immunohistochemistry. Opinion from 2 other pathologists working in the same center was also sought when required. The final diagnosis in case of any disputes was given by Dr AB.
Paraffin embedded sections, blocks for review, and decalcified bone marrow specimens were cut at three μ thickness, stained by hematoxylin and eosin stain, and examined. A provisional diagnosis was generated, which was followed by an immunohistochemical (IHC) workup to arrive at the final diagnosis. An effort was made to classify all the cases in accordance with the prevalent WHO classification. Few cases could not be grouped into any specific subtype, the reasons being suboptimal fixation, poor morphology, poor antigen preservation, or scant tissue, which could not be further worked up. These cases were classified as high-grade, low-grade, or unclassified B- or T-cell lymphoma, respectively.
A panel of IHC markers was used based on the morphological diagnosis. The antibodies commonly used were CD45 (LCA), CD79a, CD20, CD19, CD23, CD10, CD3, CD5, CD2, CD7, CD56, CD4, CD8, CD15, CD30, CD34, CD21, CD23, CD68, MUM1, BCL6, TdT, BCL2, MIB1/Ki-67, ALK1, C-MYC, LEF1, BOB1, OCT2, ICOS, PD-1, Granzyme B, TIA-1, and EBER-ISH. Other IHC markers were also employed as and when required. IHC was performed on Ventana and Dako automated IHC platforms.
RESULTS
There were a total of 2543 cases, out of which 2505 cases were included in the present study. 38 cases with poor quality blocks, depleted tissue in the blocks and non-representative biopsy could not be evaluated any further and were excluded from the present study.
The present study had a total of 2505 lymphoma cases, ranging from 3 years to 92 years of age. The majority of the cases belonged to the 5th decade.
HL constituted 22.5% (n: 563), and NHL constituted 77.5% (n: 1942). Amidst the HL cases, CHL accounted for 86.3% (n: 486), and NLPHL accounted for 13.7% (n: 77). Whereas among the NHLs, B-cell/gray zone lymphoma (GZL) accounted for 85% (n: 1650) and T/NK cell neoplasms for 15% (n: 292). GZL accounted for 0.5% of cases (n: 11). The overall distribution of the total lymphomas is shown in Figure 1.

- Broad subdivision of lymphomas in the present study. CHL: Classical Hodgkin Lymphoma, NLPHL: Nodular Lymphocyte predominant Hodgkin Lymphoma, B-cell lymphoma, T-cell lymphoma.
B-cell NHL (B-NHL) constituted 1650 cases, of which mature B-cell neoplasm was 98% (n: 1618) and B-cell lymphoblastic lymphoma (B-LBL) made up 2% (n: 32). While subclassifying B-NHLs, DLBCL was the most common subtype accounting for 54.12% (n: 893). The DLBCL group included various subtypes. This included DLBCL, not otherwise specified (NOS) (n: 708, 42.9%), primary mediastinal large B-cell lymphoma (n: 44, 2.66%), plasmablastic lymphoma (n: 28, 1.69%), Epstein-Barr virus (EBV)-positive DLBCL, NOS (n: 20, 1.21%), T-cell/histiocyte rich large B-cell lymphoma (LBCL) (n: 19, 1.15%), LBCL with IRF4 rearrangements (LBCL with IRF4) (n:5, 0.3%), CD5 positive DLBCL (n: 3, 0.18%), ALK positive LBCL (ALK+LBCL) (n: 2, 0.12%), intravascular LBCL (n: 2, 0.12%), primary cutaneous (PC) diffuse LBCL, leg type (n: 2, 0.12%), DLBCL of the CNS (PCNSL) (n: 1, 0.06%), lymphomatoid granulomatosis (n: 1, 0.06%), primary effusion lymphoma (n: 1, 0.06%) and high-grade B-NHL (HG-BNHL), and NOS (n: 57, 3.45%) [Figure 2].
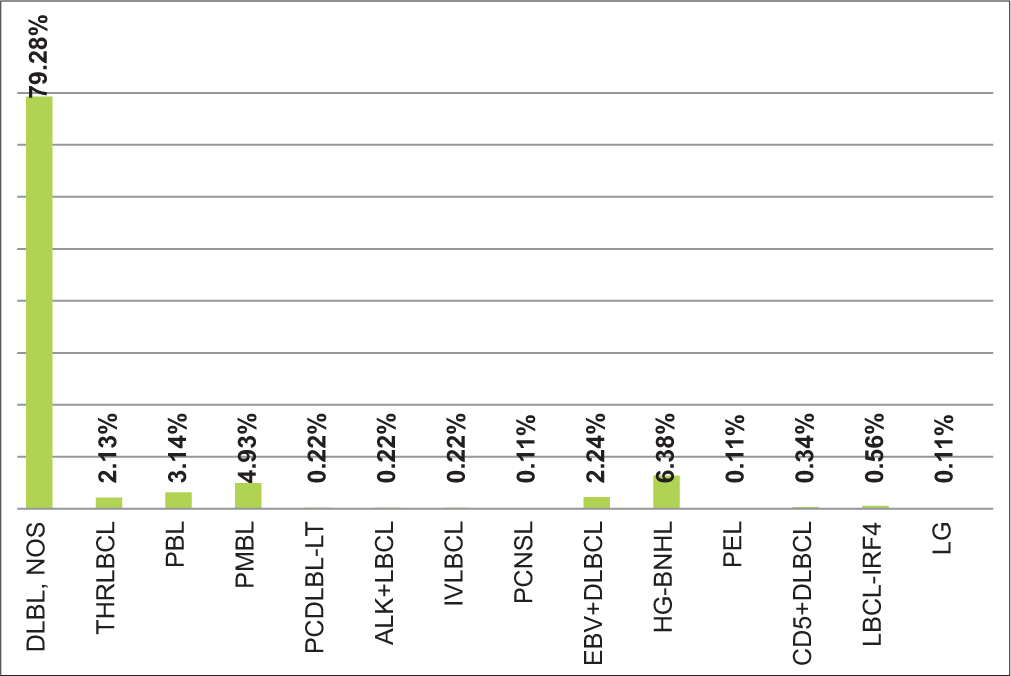
- Diffuse large B-cell lymphoma subtypes. DLBL, NOS: Diffuse large B-cell lymphoma, not otherwise specified, THRLBCL: T-cell/histiocyte rich large B-cell lymphoma, PBL: Plasmablastic Lymphoma, PMBL: Primary mediastinal large B-cell lymphoma, PCDLBL-LT: Primary cutaneous diffuse large B-cell lymphoma leg type, ALK+LBCL: ALK Positive large B-cell lymphoma, IVLBCL: Intravascular large B-cell lymphoma, PCNSL: Primary central nervous system lymphoma, EBV+DLBCL: Epstein-Barr virus positive diffuse large B-cell lymphoma, HG-BNHL: High grade B-cell non hodgkin lymphoma, PEL: Primary effusion lymphoma, CD5+DLBCL: CD5 positive diffuse large B-cell lymphoma, LBCL-IRF4: Large B-cell lymphoma with IRF4 rearrangements, LG: lymphomatoid granulomatosis.
Follicular lymphoma (FL) was the second most common NHL, accounting for 19.09% (n: 315). Grade 1/2 FL was the most common, accounting for 256 cases (81.3%), followed by grade 3A (n: 35, 11.1%) and 3B (n: 24, 7.6%).
It was followed by 97 cases (5.88%) of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); 5 (5.2%) of which showed Richter’s transformation. The other cases included Burkitt lymphoma (BL) (n: 47, 2.85%), mantle cell lymphoma (MCL) (n: 75, 4.55%), nodal marginal zone lymphoma (NMZL) (n: 27, 1.64%), extranodal marginal zone lymphoma (ENMZL) (n: 52, 3.15%), splenic marginal zone lymphoma (SMZL) (n:37, 2.24%), lymphoplasmacytic lymphoma (LPL) (n:11, 0.67%), and B-LBL (n:32, 1.94%). Of the MCL, 3 (4%) cases were classified as the blastoid variant. There were 26 cases (1.33%) of low-grade B-NHL, 16 of which involved the bone marrow. There were also 23 (1.39%) unclassified cases of B-NHL. The reasons they could not be subclassified further were suboptimal fixation and processing, scant tissue availability for further workup by IHC, and crushed biopsy in a few of the cases. There were also 3 cases (0.18%) of Hairy cell leukemia (HCL) involving the bone marrow and 1 case (0.06%) of HCL variant (HCL-v).
There were 292 cases of T/NK-cell NHL, of which mature T/NK-cell neoplasms were 80.82% (n: 236) and T-cell lymphoblastic lymphoma (T-LBL) constituted 19.18% (n: 56). The other subtypes of T-NHL included peripheral T-cell lymphoma, NOS (PTCL-NOS) (n:40, 13.7%), anaplastic large cell lymphoma (ALCL) (n: 59, 20.21%), angioimmunoblastic T-cell lymphoma (AITL) (n:69, 23.63%), nodal PTCL with T follicular helper (TFH) phenotype (n: 14, 4.79%), and extranodal NK/T-cell lymphoma (ENKTL) (n: 17, 5.82%). ALCL was further subgrouped as ALK+LBCL (n: 47) and ALK-negative ALCL (n: 12).
All cutaneous T-NHLs were included in these three groups: PC CD30+ T-cell lymphoproliferative disorder (PC-CD30+LPD) (n:12, 4.11%), primary cutaneous PTCL (PC-PTCL) (n:8, 2.74%), and mycosis fungoides (MF) (n:3, 1.03%). Within the 12 cases of PC-CD30+LPD, eight cases were of primary cutaneous ALCL (PC-ALCL). There was also one case of PC gamma delta TCL among the eight cases of PC-PTCL.
There were a few cases of hepatosplenic T-cell lymphoma (HSTCL) (n: 5, 1.71%) and subcutaneous panniculitis-like T-cell lymphoma (SPTCL) (n: 3, 1.03%). Some rare cases in this data also included two cases of follicular T-cell lymphoma (0.68%), one case (0.34%) each of adult T-cell lymphoma/leukemia (ATLL), T-prolymphocytic leukemia (T-PLL), monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL), and intestinal T-cell lymphoma, NOS. The histological subtypes of NHL are shown in Figure 3.
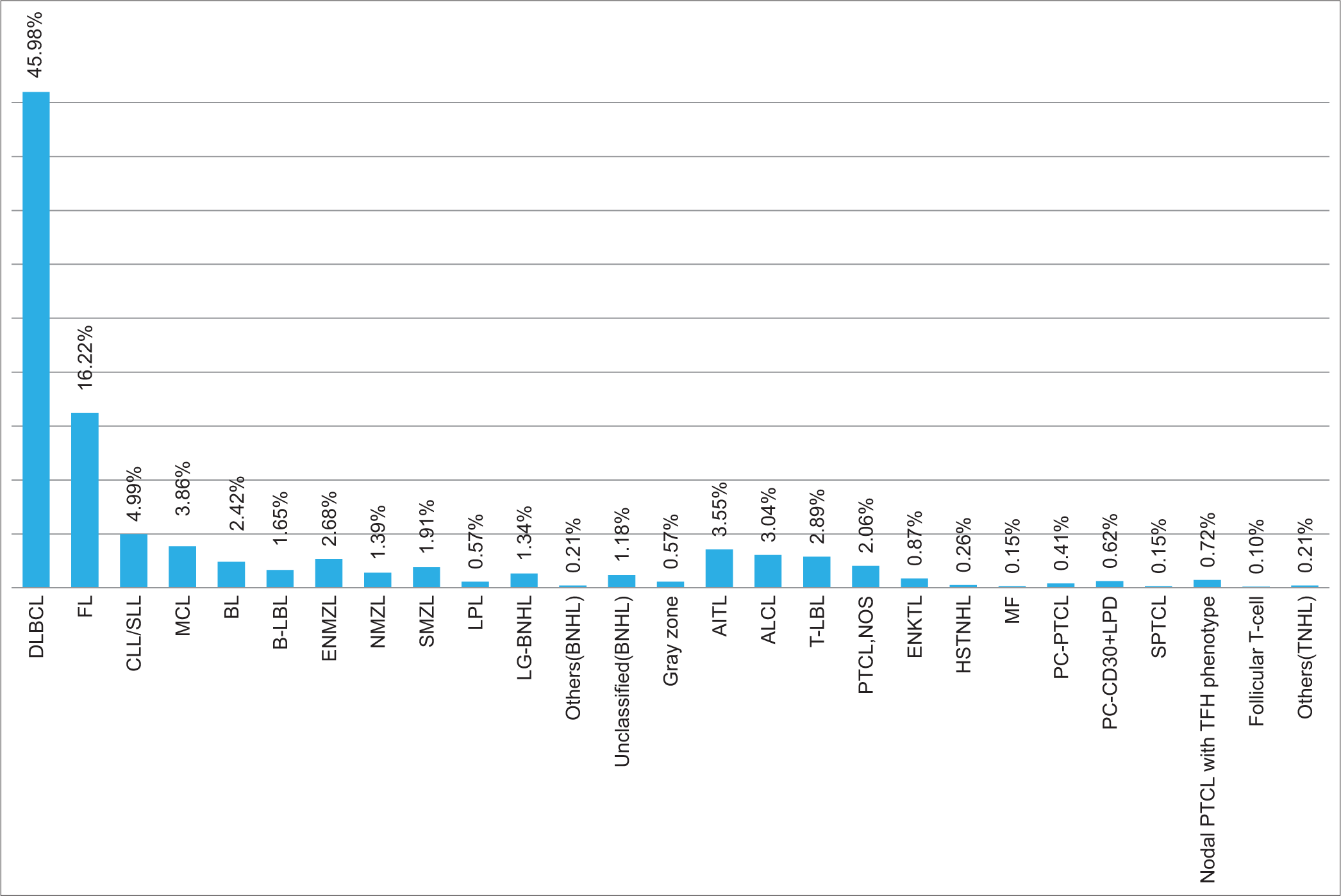
- Histological subtypes of non-Hodgkin lymphoma (n: 1942). DLBCL: Diffuse large B-cell lymphoma, FL: Follicular Lymphoma, CLL/SLL: Chronic Lymphocytic Leukaemia/Small lymphocytic Lymphoma, MCL: Mantle cell lymphoma, BL: Burkitt lymphoma, B-LBL: B-cell lymphoblastic lymphoma, ENMZL: Extra nodal marginal zone lymphoma, NMZL: Nodal marginal zone lymphoma, SMZL: Splenic marginal zone lymphoma, LPL: Lymphoplasmacytic lymphoma, LG-BNHL: Low grade B-cell non hodgkin lymphoma, BNHL: B-cell non hodgkin lymphoma, AITL: Angioimmunoblastic T-cell lymphoma, ALCL: Anaplastic large cell lymphoma, T-LBL: T-cell lymphoblastic lymphoma, PTCL, NOS: Primary T-cell lymphoma, not otherwise specified, ENKTL: Extranodal NK/T-cell lymphoma, HSTNHL: Hepatosplenic T-cell non hodgkin lymphoma, MF: Mycosis Fungoides, PC-PTCL: Primary cutaneous peripheral T-cell lymphoma, PC-CD30+LPD: Primary cutaneous CD30 positive lymphoproliferative disorder, SPTCL: Subcutaneous panniculitis like T-cell lymphoma, Nodal PTCL with TFH phenotype: Nodal peripheral T-cell lymphoma with T- follicular helper cell phenotype, TNHL: T-cell non hodgkin lymphoma.
DISCUSSION
The incidence of lymphomas shows substantial variation worldwide, which may be attributed to continual advancement in the understanding of the disease process along with the introduction of newer entities associated with frequent revisions of lymphoma classifications.[8] In this study, lymphomas were classified according to the revised 2016 WHO classification and the frequency of various subtypes of lymphomas was studied.
HL accounted for 22.4% of cases, which is comparable to the results from studies by Patkar et al. (20.1%), Arora et al. (21.3%), Yang et al. (13%), Smith et al. (14.4%), and Lisa et al. (19.6%).[7,9-12] The prevalence of HL in this study is higher than that documented in other Asian countries such as Thailand, China, and Japan (5.2–8%).[13-16]
NLPHL constituted 13.7%, which is comparable with an earlier study by Patkar et al. (11.9%).[7] However, this incidence is higher when compared to other studies from India, as well as Asia (2–4%).[9,12,15,16]
B-NHLs accounted for 85%, while T/NK cell lymphomas accounted for 15%, respectively, in our study. The previous studies from India by Naresh et al. (79.1% and 15.2%),[4] Sahni and Desai (79.3% and 18.8%),[8] and Arora et al. (78.6% and 20.2%),[9] also showed similar findings. The incidence of T/NK cell lymphomas was found to be much less in the West (~5%)[11,17] but was higher in other Asian countries (21–26%).[10,14,15] In the current data, GZL accounted for 0.56%, which was similar to the study by Arora et al. (0.3%) and Smith et al. (0.4%),[9,11] whereas a study from Japan found it to be only 0.06%.[16]
Precursor B-LBL accounted for 1.65%, while the frequency of T-LBL was 2.89%. The frequency of T-LBL was similar to that noted by Arora et al. (2.2%).[9] The distribution of B-LBL was similar to other studies from North and East India (2.1– 2.3%)[12,18] but was higher in number compared to some of the previous studies from India (0.2–0.6%).[4,8,9] A study from the USA found B-LBL to be 3.75%.[17]
In the present study, the DLBCL group was the most common NHL, accounting for 45.9%, respectively. World over, DLBCL constitutes approximately 30–58% of all NHL cases.[18] The present study showed similar observations, and comparable results were also noted in other studies from India, with DLBCL percentage varying from 47% to 58%.[8,9,12,18] Studies from other parts of the world show identical results, with DLBCL percentages ranging from 41% to 50%.[11,14-16]
DLBCL-NOS accounted for 36.45% of our cases. Of the 708 DLBCL-NOS cases, 635 were further subdivided into the germinal center (GC) and activated B-cell (ABC) subtypes using the Hans algorithm. The patterns of expression of CD10, BCL6, and MUM1 were studied for this subtyping. Among these 635 cases, GC type accounted for 38.2%, while ABC type accounted for 61.8%. In the study by Hans’ et al., GC type accounted for 42%, and ABC type accounted for 58%.[19] Similar findings were also found in a study from China, with GC type accounting for 31.2% and ABC type 68.8%.[15] This is important prognostically, as the ABC subtype is supposed to have a poor prognosis compared to the GC type.[19] Along with the aforementioned IHC markers, C-MYC and BCL2 were also done, with MYC and BCL2 coexpressing tumors (with the threshold of 40% and 50%, respectively) classified as double expressor (DE). In our study, 263 cases (37.14%) were DE, of which 73% belonged to the ABC subtype. Hashmi et al.[20] also found similar results with 35.8%, whereas Naseem et al.[21] found the frequency of DE to be 14.8%. They also found a significant association of DE phenotype with the ABC type, which is similar to our results. Overall, this phenotype and its association with ABC type confer a poorer prognosis to these patients. In our study, the cases with DE phenotype and of GC subtype by Hans’ algorithm were advised cytogenetic studies to assess for rearrangements of MYC and BCL2/BCL6 genes to establish the diagnosis of double-hit/triple-hit lymphomas.
The broad category of DLBCL included various other common and rare subtypes. Here, we have reported their incidence and also compared them with other data available from the rest of India and world literature, as shown in Table 1.
| Present study | Arora et al.[9] India |
Naresh et al.[4] India |
Sahni and Desai[8] India | Jung et al.[13] Korea |
Muto et al.[16] Japan |
Sukpanichnant[14] Thailand |
Smith et al.[11] UK |
|
|---|---|---|---|---|---|---|---|---|
| THRLBCL (%) | 0.9 | 1.2 | - | - | 0.19 | - | - | 0.6 |
| PMBL (%) | 2.26 | 0.6 | 0.2 | 0.1 | 1.02 | 0.51 | 0.87 | - |
| PBL (%) | 1.44 | 0.4 | - | - | 0.39 | 0.14 | - | - |
| HG-BNHL (%) | 2.93 | - | 6.1 | 0.9 | 0.58 | 1.57 | - | - |
| PEL (%) | 0.05 | - | - | - | 0.15 | - | 0.16 | - |
| LG (%) | 0.05 | 0.2 | - | - | 0.1 | 0.03 | - | - |
| PCLBCL-LT (%) | 0.1 | - | - | - | 0.04 | - | - | - |
| ALK+LBCL (%) | 0.1 | 0.05 | - | - | 0.01 | - | - | - |
| IVLBCL (%) | 0.1 | - | - | - | 0.20 | 0.35 | 0.93 | - |
| PCNSL (%) | 0.05 | - | - | - | 3.8 | - | - | - |
| EBV+DLBL, NOS (%) | 1.02 | - | - | - | 0.9 | - | - | - |
| CD5+DLBL (%) | 0.15 | 0.03 | - | - | - | - | - | - |
| LBCL-IRF4 (%) | 0.25 | - | - | - | - | - | - | - |
DLBCL: Diffuse large B-cell lymphoma, NOS: Not otherwise specified, ALK+LBCL: ALK positive large B-cell lymphoma, PBL: Plasmablastic lymphoma, THRLBCL: T-cell/histiocyte rich large B-cell lymphoma, PEL: Primary effusion lymphoma, IVLBCL: Intravascular large B-cell lymphoma, PCNSL: Primary DLBCL of the CNS, EBV: Epstein-Barr virus, PMBL: Primary mediastinal large B-cell lymphoma, HG-BNHL: High-grade B-cell non-Hodgkin lymphoma, LG: Lymphomatoid granulomatosis, , PCLBCL-LT: Primary cutaneous large B-cell lymphoma leg type, DLBL: Diffuse large B-cell lymphoma, LBCL: Large B-cell lymphoma
Studies on LBCL with IRF4 rearrangement were rare. We had five cases in our study; however, the largest case study was from Germany[22], which identified 20 cases with IRF4 rearrangement.
HG-BNHL comprised lymphomas that replaced “B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL.” They account for 3% of B-NHLs which are similar to that found in our study.[23] PCNSL, in our study, was only 0.05%, which is supported by the literature, where it is said to account for <1% of all NHL.[24]
FL was the second most common NHL, accounting for 16.22%, which was slightly higher compared to other Indian studies by Naresh et al. (12.6%) and Sahni and Desai (13.1%).[4,8] A recent study from Japan also found an increasing prevalence of FL, with 6% in 1996–2000 and reaching 22.4% in 2007–2014.[16] Grade 1/2 of FL accounted for 256 cases (81%), followed by grades 3A (n: 35, 11%) and 3B (n: 24, 7.6%). It is important clinically to differentiate grades 1/2 and grade 3A from grade 3B, as the treatment is more aggressive for grade 3B lymphomas, whereas grade 1/2 behaves relatively in an indolent fashion.[25]
Compared to the West, the incidence of CLL/SLL was lower in India (4.1–5.6%)[4,8,9] and other southeast Asian countries (1–6%),[10,14,16] with our data accounting for 4.9%. This could be accounted for by the fact that most cases of CLL/SLL are diagnosed on peripheral blood and may not have undergone a formal lymph node biopsy. Richter transformation was seen in 5.15% of cases of CLL in our study. It represents an aggressive transformation of CLL and is known to occur in around 5–10% of patients, which correlates with the findings in our study.[26]
BL accounted for 2.42% of the total NHLs, which is similar to other Indian studies (1.8–3%).[4,8] Studies from China[10] and the UK[11] also had similar results (1.9–2.1%). The frequencies of MCL, NMZL, ENMZL, and LPL in the present study were similar to the findings in the previous studies from India.[4,8,9]
SMZL accounted for 1.9% of cases, which is higher compared to other studies.[8,9] This may be due to the fact that we receive a fair number of bone marrow biopsy cases for primary diagnosis. SMZL generally accounts for <2% of all lymphoid malignancies;[27] hence, our data is still relevant to the current scenario.
HCL and HCL-v accounted for 0.15% and 0.05% of cases, respectively. The frequency of HCL is similar to that found by Sahni and Desai (0.1%).[8] HCL-v was not reported in any of the previous studies from India.
PTCL-NOS accounted for 2.05% of cases. The frequency of PTCL-NOS is seen to vary from 1.9 to 4.6% in various studies from India.[4,8,28] Few studies from the west[11,17] also showed similar results.
The frequency of ALCL was 3.04%, which was similar to other studies from India by Nair et al. (3.1%), Burad et al. (3.9%), and Naresh et al. (4%).[4,28,29] Similar findings were also noted in studies from Thailand (3.6%) and China (3.5%).[10,14] ALCL cases were further subgrouped as ALK-positive and ALK-negative, where ALK-positive cases accounted for 2.4% and ALK-negative for 0.6%. Nair et al.[28] had 1.8% of ALK-positive cases. However, in the study by Nemani et al.,[30] ALK-negative ALCL was more frequent. Under the umbrella term of “nodal T-cell lymphomas of follicular T helper phenotype,” AITL added up to 3.55% in our data set. There has been a significant improvement in the ability to make this diagnosis in recent times with the availability of new IHC markers. The previous studies from India had fewer cases as compared to our data (0.4–1%);[4,8] however, more recent studies by Nair et al. (3.28%)[28] and Nemani et al. (2.88%)[30] found results similar to study. Other studies from China (3.3%)[10] and Korea (2.6%)[13] also showed results that were similar to our data. A study from Japan,[16] however, found AITL to be of slightly higher frequency (5.3%). Our study also had 14 cases of nodal PTCL with TFH phenotype and two cases of follicular T-cell lymphoma. In a study from Korea,[31] old cases were reviewed, and among 207 cases, 111 had AITL, 67 had PTCL-NOS, 19 had F-PTCL, and 10 had nodal PTCL with TFH phenotype. A single case of follicular T-cell lymphoma was also reported by Lisa et al. from India.[12] Other than this, there are no other studies from India of these entities, to the best of our knowledge.
The frequency of ENKTL was 0.87% in our data, which was similar to other studies from India (0.7–1%),[4,8,9] whereas the frequency of ENKTL in two studies from China was considerably high, accounting for 13.4% and 17%, respectively.[10,15]
SPTCL accounted for 0.15% of cases. Similar frequency was also observed in other Indian studies (0.1–0.2%).[4,28] Cases of HSTCL constituted 0.26%, which contrasted with the study by Arora et al. (0.5%)[9] and also with other Indian studies (0.01–0.04%).[4,28]
PC-CD30+ LPD formed 0.6% of all NHLs, 0.4% of which were PC-ALCL, which was very similar to that documented by Nair et al.[28] and Burad et al.[29] Similar results were also noted in foreign literature.[10,11] PC-PTCL constituted 0.4%, similar to that documented by Sahni and Desai (0.2%)[8] but higher than other Indian studies (0.04– 0.05%).[9,28] MF constituted a small subset in our study with results similar to literature from China (0.25%)[10] and Japan (0.18%),[16] but much less compared to other Indian studies.[4,8,9]
The other rare subtypes included one case each of ATLL, T-PLL, MEITL, and intestinal T-cell lymphoma, NOS. Similar results of T-PLL and ATLL were found by Arora et al.[9] but ATLL was fairly high in a study from Japan (7.96%)[16] [Tables 2 and 3].[32]
| Entity | SEEU (%) | CSA (%) | NA/ME/IN (%) | SAF (%) | FE (%) | Developing region (%) (SEEU+CSA+NA/ME/IN+SAF+FE) | Developed region (%) (North America+Western Europe) | Present study (%) |
|---|---|---|---|---|---|---|---|---|
| DLBCL, NOS | 38.8 | 39 | 47.2 | 36.3 | 48.6 | 42.5 | 28.9 | 36.45 |
| FL | 15.8 | 20.7 | 12.4 | 18.1 | 9.4 | 15.3 | 25.5 | 16.22 |
| CLL/SLL | 11.3 | 3.1 | 7 | 8.4 | 2.7 | 6.1 | 7 | 4.99 |
| MALT | 6.6 | 7 | 2.7 | 2.5 | 6.8 | 5.2 | 8.8 | 2.68 |
| MCL | 5.9 | 5 | 2.2 | 1.8 | 3.6 | 3.8 | 7.8 | 3.86 |
| MZL (NMZL+SMZL) | 3.7 | 2.6 | 2 | 1.8 | 2.2 | 2.5 | 3 | 3.3 |
| BL | 1.5 | 3 | 2.7 | 1.6 | 1.8 | 2.2 | 0.8 | 2.42 |
| PMBL | 2.4 | 1.5 | 2.2 | 1.8 | 1.9 | 2 | 2.1 | 2.26 |
| HG-BNHL | 0.7 | 0.9 | 3.6 | 3.3 | 1 | 1.9 | 0.9 | 2.93 |
| LG-BNHL | 1.2 | 0.7 | 1.1 | 0.6 | 0.7 | 0.9 | 1.2 | 1.3 |
| B-LBL | 0.7 | 2.4 | 1.1 | 0.2 | 0.4 | 1.1 | 0.3 | 1.65 |
| LPL | 0.5 | 0.2 | 0.1 | 0.8 | 0.3 | 0.3 | 1.4 | 0.56 |
| PTCL, NOS | 2.7 | 2.5 | 2.7 | 6.4 | 4.6 | 3.5 | 2.6 | 2.06 |
| T-LBL | 1.5 | 1.5 | 4 | 3.7 | 3.7 | 2.9 | 1.3 | 2.89 |
| ENKTL | 0.8 | 3 | 1.1 | 0.4 | 5.2 | 2.2 | 0.3 | 0.87 |
| ALCL | 1 | 1.5 | 3.3 | 1.8 | 1.9 | 2 | 2.2 | 3.04 |
| AITL | 1.5 | 1.4 | 0.7 | 0.4 | 2.5 | 1.3 | 1.2 | 3.55 |
| MF | 0.7 | 0.4 | 0.7 | 0.4 | 0 | 0.4 | 1 | 0.15 |
| ATLL | 0 | 1.1 | 0 | 0 | 0 | 0.3 | 0.1 | 0.05 |
| PTCL, other types | 0.7 | 1.4 | 0.7 | 1 | 0 | 0.8 | 0.5 | 2.37 |
| Total no. of cases | 1137 | 1712 | 1663 | 905 | 1225 | 6642 | 1867 | 1942 |
SEEU: Southeastern Europe, CSA: Central/South America, NA/ME/IN: North Africa/Middle East/India, SAF: Southern Africa, FE: Far East, PMBL: Primary mediastinal large B-cell lymphoma, DLBCL: Diffuse large B-cell lymphoma, NOS: Not otherwise specified, FL: Follicular lymphoma, BL: Burkitt lymphoma, MZL: Marginal zone lymphoma, NMZL: Nodal marginal zone lymphoma, SMZL: Splenic marginal zone lymphoma, LPL: Lymphoplasmacytic lymphoma, MCL: Mantle cell lymphoma, ALCL: Anaplastic large cell lymphoma, AITL: Angioimmunoblastic T-cell lymphoma, ENKTL: Extranodal NK/T-cell lymphoma, PTCL, NOS: Peripheral T-cell lymphoma, NOS, MF: Mycosis fungoides, ATLL: Adult T-cell lymphoma/leukemia, HG-BNHL: High-grade B-cell non-Hodgkin lymphoma, LG-BNHL: Low-grade B-cell non-Hodgkin lymphoma, B-LBL: B-cell lymphoblastic lymphoma, T-LBL: T-cell lymphoblastic lymphoma, PMBL: Primary mediastinal large B-cell lymphoma, MALT: Mucosa associated lymphoid tissue, CLL/SLL: Chronic lymphocytic leukemia/small lymphocytic lymphoma.
| Entity | Present study (%) | Naresh et al.[4] (%) India |
Sahni and Desai[8] (%) India | Arora et al.[9] (%) India |
Sukpanichnant[14] Thailand[14] (%) | Jung et al.[13] Korea (%) | Ekström-Smedby[2] Japan (%) | Meng et al.[15] China (%) |
Smith et al[11] UK (%) |
Morton et al.[17] USA (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| B-LBL | 1.65 | 0.6 | 0.2 | 0.4 | 0.87 | 0.7 | 0.18 | 5.2 | - | 3.75 |
| T-LBL | 2.89 | 6.06 | 6.9 | 2.21 | 4.10 | 1.49 | 0.68 | - | 1.09 | |
| DLBCL-NOS | 36.45 | 33.8 | 50.2 | 46.9 | 50.4 | 36.7 | 39.94 | 41.2 | 47.83 | 31.29 |
| FL | 16.22 | 12.6 | 13.1 | 10.9 | 8.37 | 7.5 | 24.8 | 5.8 | 18.6 | 13.81 |
| BL | 2.42 | 1.8 | 3.0 | 3.4 | 4 | 1.75 | 0.77 | 1.91 | 2.07 | 1.42 |
| CLL/SLL | 4.99 | 5.6 | 5.4 | 4.1 | 2.57 | 1.21 | 1.23 | 4.61 | - | 21.91 |
| MCL | 3.86 | 3.4 | 2.1 | 1.6 | 1.04 | 2.25 | 3.12 | 3.15 | 4.97 | 2.18 |
| NMZL | 1.39 | 1.9 | 0.2 | 0.8 | 0.27 | 1.47 | 0.68 | 0.09 | 19.8 | - |
| ENMZL | 2.68 | 6.1 | 2.7 | 2.4 | 4.16 | 19.9 | 4.12 | 6.3 | 4.19 | |
| SMZL | 1.9 | 0.2 | 0.5 | 0.4 | 0.32 | 0.1 | 0.27 | 0.35 | - | |
| LPL | 0.57 | 0.6 | 0.1 | 1.7 | 0.82 | 0.28 | 0.49 | 0.79 | - | 2.65 |
| AITL | 3.55 | 1.0 | 0.4 | 1.4 | 1.8 | 2.57 | 5.26 | 3.33 | 1.1 | 0.23 |
| ALCL | 3.04 | 4.1 | 4.8 | 5.1 | 3.66 | 1.88 | 1.26 | 3.53 | 0.92 | 1.11 |
| ENKTL | 0.87 | 0.7 | 1.1 | 0.9 | - | 4.24 | 0.75 | 17.1 | - | - |
| PTCL-NOS | 2.06 | 1.9 | 4.6 | 5.9 | 13.1 | 3.43 | 3.45 | 3.99 | 1.83 | 3.27 |
| PC-PTCL | 0.4 | - | - | - | - | - | 0.25 | - | - | - |
| PCCD30+LPD | 0.6 | 0.1 | 0.2 | 0.05 | - | 0.63 | 0.3 | 0.67 | 0.74 | - |
| MF | 0.15 | 0.9 | 0.6 | 2.2 | 1.25 | 0.9 | 0.18 | 0.25 | 0.78 | 2.29 |
| HSTCL | 0.26 | 0.01 | - | 0.6 | 0.16 | 0.02 | 0.02 | 0.25 | - | - |
| SPTCL | 0.15 | 0.1 | - | 1.1 | 0.49 | 0.31 | 0.05 | 0.97 | - | - |
B-LBL: B-cell lymphoblastic lymphoma, T-LBL: T-cell lymphoblastic lymphoma, DLBCL: Diffuse large B-cell lymphoma, NOS: Not otherwise specified, FL: Follicular lymphoma, BL: Burkitt lymphoma, CLL/SLL: Chronic lymphocytic leukemia/small lymphocytic lymphoma, NMZL: Nodal marginal zone lymphoma, ENMZL: Extranodal marginal zone lymphoma, SMZL: Splenic marginal zone lymphoma, LPL: Lymphoplasmacytic lymphoma, MCL: Mantle cell lymphoma, ALCL: Anaplastic large cell lymphoma, AITL: Angioimmunoblastic T-cell lymphoma, ENKTL: Extranodal NK/T-cell lymphoma, PTCL-NOS: Peripheral T-cell lymphoma-Not otherwise specified, PC-PTCL: Primary cutaneous-Peripheral T-cell lymphoma, PC-CD30+LPD: Primary cutaneous CD30+T-cell lymphoproliferative disorder, MF: Mycosis fungoides, HSTCL: Hepatosplenic T-cell lymphoma, SPCTL: Subcutaneous panniculitis-like T-cell lymphoma
CONCLUSION
The majority of the entities in the WHO classification can be diagnosed and subclassified based on morphology, combined with IHC and relevant clinical details. In our study, one of the highlights is the subtyping of DLBCL-NOS into GC (38.2%) and ABC type (61.8%) by Hans’ algorithm, which is important for prognostication. Here, we also emphasize that the ABC type has a stronger association with the DE phenotype. Various other rare large B-cell lymphomas have also been documented. Furthermore, due to the availability of newer IHC markers (ICOS, PD1, Granzyme B, TIA1), proper categorization of T-cell lymphomas according to the latest WHO classification was possible. ISH for EBERs was also done to identify lymphomas that were EBV positive, thereby determining their prognosis and treatment. Of all the NHLs, the most common was DLBCL followed by FL in our data set. Besides these findings, the overall incidence of HL and NHL in the current study was fairly comparable to the rest of the country and the world.
Ethical approval
Institutional Ethics Committee approval is not required as this is a retrospective study looking at previously diagnosed cases in the institute. There was no direct patient intervention done in the study. All the data was anonymized.
Declaration of patient consent
Patient consent was not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Health disparities and the global landscape of lymphoma care today. Am Soc Clin Oncol Educ Book. 2017;37:526-34.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and etiology of non-Hodgkin lymphoma--a review. Acta Oncol. 2006;45:258-71.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic alterations in B cell lymphoma subtypes as potential biomarkers for noninvasive diagnosis, prognosis, therapy, and disease monitoring. Turk J Biol. 2020;44:1-14.
- [Google Scholar]
- Distribution of various subtypes of non-Hodgkin's lymphoma in India: A study of 2773 lymphomas using R.E.A.L. and WHO Classifications. Ann Oncol. 2000;11(Suppl 1):63-7.
- [CrossRef] [PubMed] [Google Scholar]
- Diffuse large B-cell lymphoma: A retrospective study from a regional care center in South India. Indian J Cancer. 2018;55:66-9.
- [CrossRef] [PubMed] [Google Scholar]
- Global, regional, and national burden of Hodgkin lymphoma from 1990 to 2017: Estimates from the 2017 Global Burden of Disease study. J Hematol Oncol. 2019;12:107.
- [CrossRef] [PubMed] [Google Scholar]
- Immunoprofile of Hodgkin's lymphoma in India. Indian J Cancer. 2008;45:59-63.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution and clinicopathologic characteristics of non-Hodgkin's lymphoma in India: A study of 935 cases using WHO classification of lymphoid neoplasms (2000) Leuk Lymphoma. 2007;48:122-33.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency and distribution of lymphoma types in a tertiary care hospital in South India: Analysis of 5115 cases using the World Health Organization 2008 classification and comparison with world literature. Leuk Lymphoma. 2013;54:1004-11.
- [CrossRef] [PubMed] [Google Scholar]
- Subtype distribution of lymphomas in Southwest China: Analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6:77.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphoma incidence, survival and prevalence 2004-2014: Sub-type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575-84.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of lymphoma subtypes in Bihar-analysis of 518 cases using the WHO classification of lymphoid tumors (2017) J Lab Physicians. 2020;12:103-10.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of malignant lymphoma subtypes in Korean patients: A report of the 4th nationwide study. J Hematopathol. 2019;12:173-81.
- [CrossRef] [Google Scholar]
- Analysis of 1983 cases of malignant lymphoma in Thailand according to the World Health Organization classification. Hum Pathol. 2004;35:224-30.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiologic characteristics of malignant lymphoma in Hubei, China: A single-center 5-year retrospective study. Medicine (Baltimore). 2018;97:e12120.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and secular trends of malignant lymphoma in Japan: Analysis of 9426 cases according to the World Health Organization classification. Cancer Med. 2018;7:5843-58.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265-76.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical (IHC) analysis of non-Hodgkin's lymphoma (NHL) spectrum according to WHO/REAL classification: A single centre experience from Punjab, India. J Clin Diagn Res. 2014;8:46-9.
- [Google Scholar]
- Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275-82.
- [CrossRef] [PubMed] [Google Scholar]
- Double-expressor phenotype (BCL-2/cMYC Co-expression) of diffuse large B-cell lymphoma and its clinicopathological correlation. Cureus. 2021;13:e13155.
- [CrossRef] [Google Scholar]
- The Frequency of double expresser in selected cases of high grade diffuse large b-cell lymphomas. Asian Pac J Cancer Prev. 2020;21:1103-7.
- [CrossRef] [PubMed] [Google Scholar]
- Translocations activating IRF4 identify a subtype of germinal center-derived B-cell lymphoma affecting predominantly children and young adults. Blood. 2011;118:139-47.
- [CrossRef] [PubMed] [Google Scholar]
- High-grade B-cell lymphomas, not otherwise specified: A study of 41 cases. Cancer Manag Res. 2020;12:1903-12.
- [CrossRef] [PubMed] [Google Scholar]
- Rare cases of primary central nervous system anaplastic variant of diffuse large B-cell lymphoma. Diagn Pathol. 2019;14:45.
- [CrossRef] [PubMed] [Google Scholar]
- Follicular non-Hodgkin lymphoma grades 3A and 3B have a similar outcome and appear incurable with anthracycline-based therapy. Ann Oncol. 2011;22:1164-9.
- [CrossRef] [PubMed] [Google Scholar]
- Richter's transformation in chronic lymphocytic leukemia. Oncology (Williston Park). 2012;26:1146-52.
- [Google Scholar]
- Splenic marginal zone lymphoma: From genetics to management. Blood. 2016;127:2072-81.
- [CrossRef] [PubMed] [Google Scholar]
- Profiling of peripheral T-cell lymphomas in Kerala, South India: A 5-year study. Indian J Pathol Microbiol. 2017;60:206-8.
- [CrossRef] [PubMed] [Google Scholar]
- Peripheral T-cell lymphoma: Frequency and distribution in a tertiary referral center in South India. Indian J Pathol Microbiol. 2012;55:429-32.
- [CrossRef] [PubMed] [Google Scholar]
- Peripheral T cell lymphoma: Clinico-pathological characteristics & outcome from a tertiary care centre in south India. Indian J Med Res. 2018;147:464-70.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive analysis of clinical, pathological, and genomic characteristics of follicular helper T-cell derived lymphomas. Exp Hematol Oncol. 2021;10:33.
- [CrossRef] [PubMed] [Google Scholar]
- Non-Hodgkin lymphoma in the developing world: Review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244-50.
- [CrossRef] [PubMed] [Google Scholar]







