Translate this page into:
Real World Outcomes of Check Point Inhibitors Immunotherapy in Renal and Urothelial Cancers in a Teratiary Care Cancer Center in India

*Corresponding author: Dr. Venkata Pradeep Babu, DNB, Consultant, 2nd floor A block, Rajiv Gandhi Cancer Institute and Research Centre, Sector-5, Rohini, New Delhi - 110085, India. pradeepbabu.koyyala@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Talwar V, KoyyalaVPB, Bothra SJ, Goel V, Dash PK, Jajodia A, et al. Real World Outcomes of Check Point Inhibitors Immunotherapy in Renal and Urothelial Cancers in a Teratiary Care Cancer Center in India. Int J Mol Immuno Oncol 2019;4(3):63-6.
Abstract
AIMS:
The real-world data regarding the response rates, tolerability, and toxicities of immunotherapy is very limited. The aim of this study is to analyze these characteristics in patients who have received immunotherapy for metastatic renal cell carcinoma (RCC) or urothelial cancer (UC).
Methods:
Retrospective review of patients over a year from 2017–2018 diagnosed with metastatic RCC and UC in our institute who received checkpoint inhibitors was done. PFS and OS were calculated.
Results:
A total of 16 patients, 11 with metastatic RCC and 5 patients with Metastatic UC were included in this study. All patients were male and Median age was 57.5 years. Median Number of cycles administered was 6. 50% of patients had a partial response to treatment, 16.6% of patients had stable disease and 33.3% of patients had progressive disease. There were no complete responses to therapy. Median Follow up was 9 months. The median PFS of the whole cohort was 6 months, while in RCC was 6 months and in UC was 1 month. Median OS of the whole cohort is 7 months, while the median OS for RCC and UC were 7 months and 3 months respectively. Fatigue was the most common adverse effect noted and Anaemia was the most common hematological side effect seen with immunotherapy in this study.
Conclusion:
This is real-world data of the use of the immune checkpoint inhibitors in the resource-limited setting. The benefit of Immune checkpoint inhibitors may in advanced renal cell cancers and Urothelial cancers may be different from that seen in the Western population.
Keywords
Check point inhibitors
Renal cell carcinoma
Bladder cancer
Nivolumab
Pembrolizuamb
INTRODUCTION
The immune system is the main defence mechanism against various infections and cancers. However, tumours have numerous ways of evasion of detection and eradication by the immune system. It can do so by reducing antigen expression, secreting immune-suppressive cytokines, or up-regulating inbuilt inhibitory signals. Cancer immunotherapy includes various agents, cancer immunotherapy encompasses a broad variety of agents, which can stimulate, enhance, and modulate the immune system to detect and destroy cancer cells. Immunotherapy has revolutionized the management of solid malignancies. Over the last decade, the better understanding of various intricacies of interactions between the immune system and the tumor has led to discovery of various targets and subsequently targeted therapies that can enhance the action of immune system. Th e best example for this type of therapies is modulating checkpoint receptors on T-cells (the cytotoxic T-lymphocyte antigen-4 (CTLA-4) receptor and programmed cell death 1 (PD-1) receptor) with a class of immunotherapy checkpoint inhibitors (ICIs). Th ese ICIs have shown effi cacy and improved survival for patients with multiple metastatic solid tumors, including melanoma,[1] non-small cell lung cancer[2,3], renal cell carcinoma,[4,5] and urothelial carcinoma.[6,7] However, the real-world data regarding the response rates, tolerability and toxicities is very limited. Th e aim of this study is to analyse these characteristics in the patients who have received immunotherapy for metastatic renal cell carcinoma or urothelial cancer.
MATERIAL AND METHODS
A retrospective chart review was conducted of patients diagnosed with renal cell cancer (RCC) and urothelial cancer (UC) patients during 2017–2018 from the electronic medical records of Rajiv Gandhi Cancer Institute and Research Centre, a tertiary care hospital in northern India. Patients with age > 18 years, with a diagnosis of metastatic RCC with or without prior therapy or metastatic UC with platinum refractory disease were selected. Baseline end stage renal disease and chronic liver disease (Child Pugh Score C) were excluded. A total of 16 patients receiving immunotherapy for RCC and UBC cancer were available for fi nal analysis. Patients were staged as per AJCC 8th edition TNM staging system.
STATISTICAL ANALYSIS
Data was summarized as frequencies for categorical variables and median and range values for continuous variables. Associations between categorical variables were assessed using Fisher’s exact test, when appropriate. Diff erences were considered statistically signifi cant when P < 0.05. Progression-free survival (PFS) was calculated from the start of ICI treatment until disease progression or death. All patients without progressive disease were censored at the time of last follow-up. OS was calculated from the start of ICI treatment until death. Patients lost to follow-up were censored at the point of last contact. Th e Kaplan–Meier method was used to estimate PFS and OS. Th e log-rank test and Cox proportional hazards regression were used to test for diff erences between groups. All statistical analyses were performed with SPSS Statistical Soft ware, version 23.0 (IBM_SPSS, Armonk, NY, USA).
OBSERVATIONS AND RESULTS
A total of 16 patients were included in the study, with 11 patients of metastatic RCC and 5 patients were of metastatic UBC. Th e median age of the study group was 57.5 years, with all male patients. Th e most common sites of metastasis were lung & lymph nodes (n = 10, 62.5% each), bone metastasis in 32.5% (n = 6), liver metastasis in 18.8% patients (n = 3), brain metastasis in 12.5% patients (n = 2). Of all the patients who received immunotherapy, 15 patients received nivolumab, one patient received pembrolizumab.
The ICI was used as fi rst line therapy in 12.5% patients (n = 2), whereas most patients (n = 14) received ICI as second line therapy or beyond. Median number of cycles of ICI received was 6 cycles, with 3 patients receiving 1 cycle and 7 patients receiving more than 10 cycles. Response could not be assessed in 25% patients (n = 4) as they received either 1 or 2 cycles of ICI, and response assessment was done in the other 75% patients (n = 12). Th e best response to therapy was partial response (PR) in 50% patients (n = 6), stable disease in 16.6% patients (n = 2) and progressive disease in 33.3% patients (n = 4). None of the patients had completed response to therapy.
The median follow up was 9 months (95% C.I. 6.7–11.28). The median overall survival for the whole cohort was 7 months (95% C.I. 2.36–11.63) [Figure 1], while the median OS for RCC cohort was 7 months and the median OS for Urinary Bladder Cancer was 3 months. Median progression free survival for the whole cohort of patients was 6 months (95% C.I. 2.50–9.49) [Figure 2], while Median PFS for RCC cohort was 6 months and Urinary Bladder Cancer was 1 month. All patients tolerated immunotherapy without any major side effects, both in nivolumab and pembrolizumab. Majority of these were grade 1 and 2 and do not require any drug dose adjustments or discontinuation. Fatigue was the most common side effect reported (n = 10). One patient had grade 2 haematological toxicity (anaemia) and 1 patient had grade 2 skin rash and pulmonary toxicity.
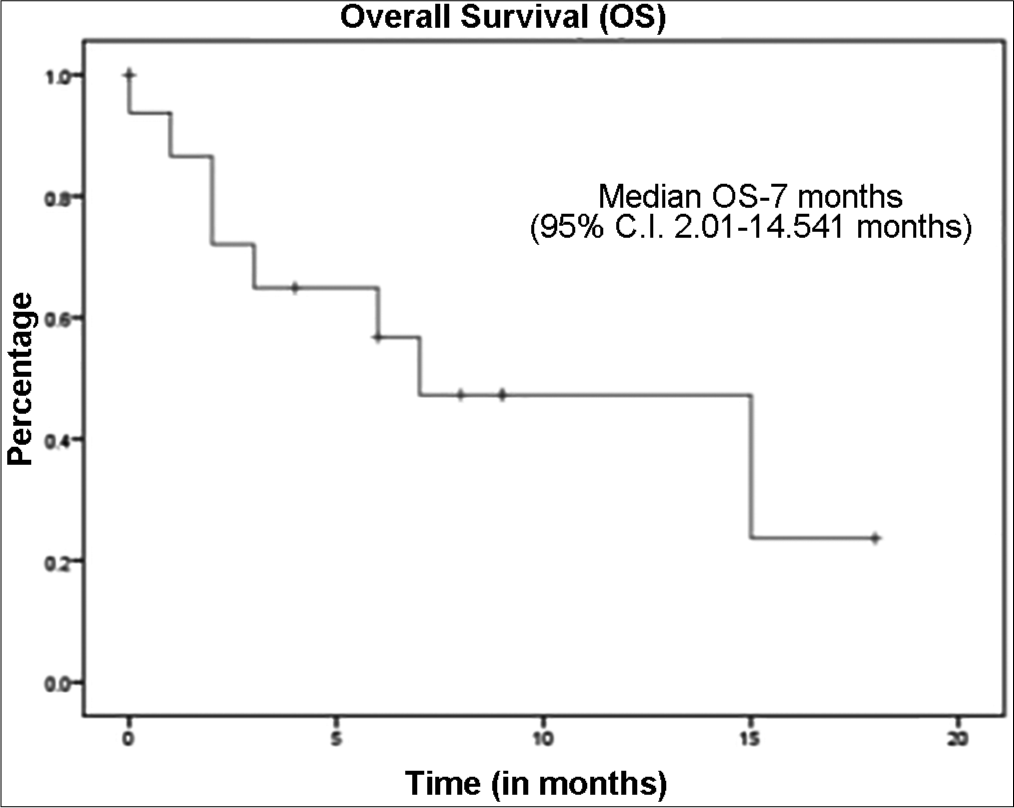
- Kaplan-Meier curve representing the overall survival of cohort.
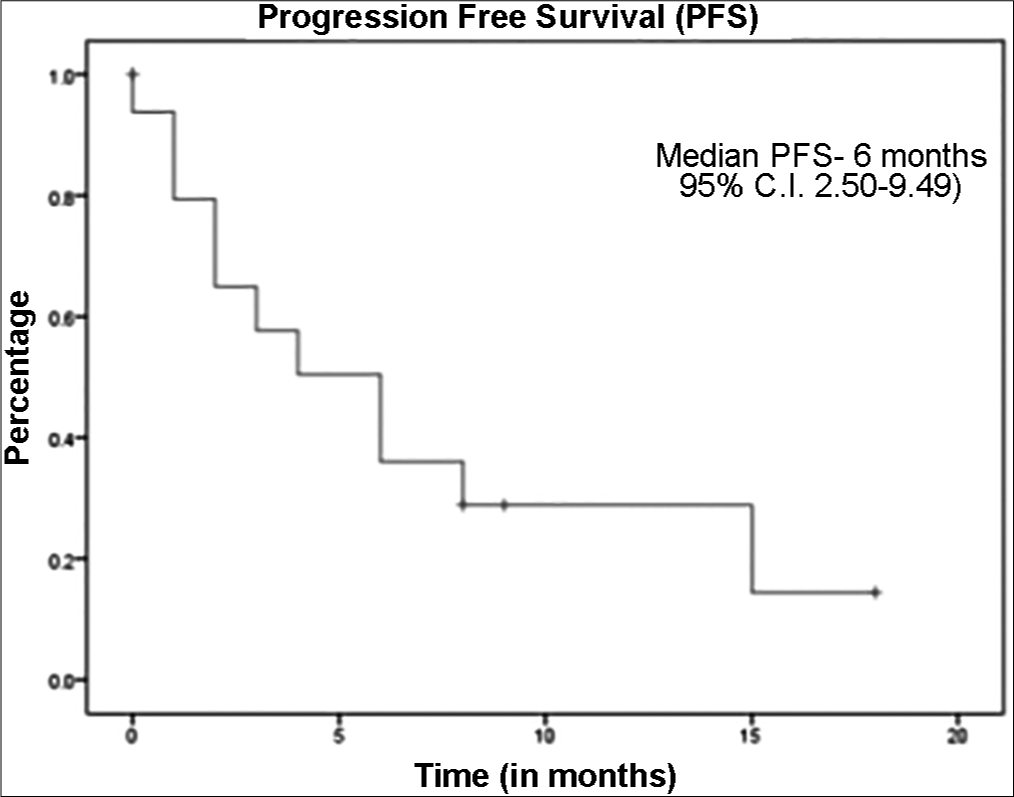
- Kaplan-Meier curve representing the progression free survival of cohort.
DISCUSSION
Immunotherapy agents broadly falls under two categories: non-specific immunotherapy and specific or directed agents. Non-specific therapy includes interferon alpha (IFN-α), various interleukins, cytokines, and vaccines.[8] In contrast, specific immunotherapy includes immune checkpoint inhibitors (ICI), which target immune checkpoints [progra mmed death 1 (PD-1), programmed death ligand 1 (PD-L1), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), lymphocyte function-associated antigen 3 (LFA-3)]. These are specific drugs (ICI) which include PD-1 inhibitors (nivolumab, pembrolizumab), PDL-1 inhibitors (Atezolizumab, Durvalumab, Avelumab), CTLA-4 inhibitors (Ipilimumab). The PD-1 and PDL-1 inhibitors induce disruption of PD-1–PD-L1 signalling, restoring the ability of T cells to selectively recognize and kill cancer cells.
Nivolumab (PD-1 inhibitor) which was approved for previously treated patients with advanced clear cell RCC based on the phase 3 Checkmate 025 study, which demonstrated OS and ORR benefits compared with Everolimus in patients who had prior antiangiogenic therapy. The PFS seen in Checkmate 025 was 4.6 months which was statistically not significantly different from that of Everolimus, while Risk of death and response rates were clearly better with Nivolumab compared to Everolimus. The median PFS seen in NIVOREN study, which aimed at seeing the real-world efficacy of Nivolumab was 3.2 months and 17% had treatment related adverse events.[9] In our study, the median PFS of mRCC cohort was 6 months, which was better than former two studies and adverse events were less, may be due to fact that the former studies included large number of patients compare to our study.
Urothelial cancer after metastasis is fatal with a median survival of 12–15 months with cisplatin based combination therapy[10] and 9 months with carboplatin based therapy.[11] In the platinum refractory setting or second line therapy, was pembrolizumab had the median OS of 10.3 months in a study by Bellmunt J et al. published in NEJM(6). In Checkmate 275 study, where Nivolumab was studied after progression on standard platinum therapy in urothelial cancer has shown median OS of 7 months. In the present study, the median OS in urothelial cancer with Nivolumab is only 1 month. However, such cross trial comparison may not be appropriate to consider that immunotherapy works differently in Indian population than in Western population, as our study is retrospective and was a retrospective study. However, the strength of this study is that it is real time data from a tertiary cancer centre which is few centres in India. These findings need evaluation in large prospective studies in Indian population.
CONCLUSION
This is a real-world data of the use of the immune checkpoint inhibitors in the resource limited setting. The benefit of immune checkpoint inhibitors may in advanced renal cell cancers and urothelial cancers may be different from that seen in Western population and we need larger studies to evaluate whether these drugs works differently in various races due to differences in genetic background constituting our immune system.
Acknowledgement
We acknowledge the support by our institution Rajiv Gandhi Cancer Institute and Research Center, New Delhi.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare no conflict of interest.
References
- Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. 2017. [cited 2019 Apr 27]. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1709684
- [CrossRef] [Google Scholar]
- Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial-The Lancet. [cited 2019 Apr 27]. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(15)01281-7/fulltext
- [Google Scholar]
- Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet Lond Engl. 2017;389(10066):255-65.
- [CrossRef] [Google Scholar]
- Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. 2015. [cited 2019 Apr 27]. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1510665
- [CrossRef] [Google Scholar]
- Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. 2018. N Engl J Med. [cited 2019 Apr 27]; Available from: https://www.nejm.org/doi/10.1056/NEJMoa1712126
- [Google Scholar]
- Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. 2017. [cited 2019 Apr 27]. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1613683
- [CrossRef] [Google Scholar]
- Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. The Lancet. 2018;391(10122):748-57.
- [CrossRef] [Google Scholar]
- Immunotherapy in genitourinary malignancies. J Hematol Oncol. 2017;10(1):95.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of nivolumab in metastatic renal cell carcinoma (mRCC): Final analysis from the NIVOREN GETUG AFU 26 study.
- Molecular biology and targeted therapies for urothelial carcinoma. Cancer Treat Rev. 2015;41(4):341-53.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized Phase II/III Trial Assessing Gemcitabine/ Carboplatin and Methotrexate/Carboplatin/Vinblastine in Patients with Advanced Urothelial Cancer Who Are Unfit for Cisplatin-Based Chemotherapy: EORTC Study 30986. J Clin Oncol. 2011;30(2):191-9.
- [CrossRef] [PubMed] [Google Scholar]






