Translate this page into:
Hyperthermic intraperitoneal chemotherapy in primary peritoneal cancer: Our experience in a young male

*Corresponding author: Rahul Sud, Department of Oncology, Command Hospital Air Force, Bengaluru, Karnataka, India. drrahulsud@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Sud R, Patra AK, Jaiswal P, Mohan R, Sindhu PK. Hyperthermic intraperitoneal chemotherapy in primary peritoneal cancer: Our experience in a young male. Int J Mol Immuno Oncol 2021;6(3):140-4.
Abstract
Primary peritoneal carcinomatosis (PPC) is a rare tumor, described in the literature almost exclusively in women. Patients with peritoneal carcinomatosis were considered incurable with low survival rates. This underwent a paradigm shift with hyperthermic intraperitoneal chemotherapy (HIPEC) after optimal cytoreductive surgery which changed the entire scenario. This case report describes the management of a 28-year-old male patient who was diagnosed to have PPC when he presented with massive ascites, who underwent cytoreductive surgery combined with HIPEC in our hospital. This procedure was complex for both the surgical team due to an extensive surgery, but also the anesthetist during the hyperthermic phase where the chemotherapy was administered. The post-operative recovery in such a case is also many times stormy and requires extreme vigilance. We had major challenges such as prolonged surgery, massive blood loss, temperature management, maintaining adequate urine output, and post-operative critical care. Extensive pre-operative preparation and proper coordination with the multidisciplinary team led us to handle the condition satisfactorily. The PPC in a young patient itself is a rare which enthuses us to report the case.
Keywords
Primary peritoneal carcinomatosis
Hyperthermic intraperitoneal Chemotherapy
cytoreductive surgery
Oncological challenges
Mismatch repair deficient
INTRODUCTION
Primary peritoneal carcinoma (PPC) is a rare malignancy and thought to be exclusively seen in females due to its close association to ovarian cancer.[1] PPC was considered an incurable since a long time due to its extensive involvement during presentation and treatment was only with palliative intent. With optimal surgical removal of the visible tumor combined with locoregional heated chemotherapeutic drugs, provide better disease control, improve quality of life and survival.[2,3] Literatures on such advanced technology are available in ovarian cancer but in PPC and that too in a young male are limited. This article summarizes the surgical, anesthetic, and oncological challenges observed in such an extensive procedure. The hunt for newer targetable driver mutation on the resected specimen in the era of immuno-oncology is also important.
CASE REPORT
A 28-year-old male, known case of extrahepatic portal obstruction on medical management since the past 2 years, reported with gradually increasing pain abdomen, abdominal distension, and weight loss for 4 months. On evaluation, the patient had gross ascites requiring large volume paracentesis every 3 days. Radiologically, the patient had multiple peritoneal deposits involving the omentum, undersurface of diaphragm, urinary bladder, and spleen. There was no other primary site detected on extensive evaluation but the CA 125 (840IU) was raised. A laparoscopic biopsy from the peritoneum was performed. The biopsy was suggestive of a poorly differentiated adenocarcinoma and further IHC on the histopathological specimen showed positivity for CK7, WT1, and ER; the patient was diagnosed to have PPC. Upper and lower GI endoscopy was normal. He received 6 cycles of chemotherapy with paclitaxel and carboplatin every 3 weekly as a neoadjuvant followed by a WBPET CT which showed a significant reduction in the ascites and a partial response to the other preexisting lesions. A cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) was planned. The patient had no comorbidities. A detailed pre-anesthetic evaluation revealed issues such as intra-abdominal mass, splenomegaly, ascites, and thrombocytopenia (Plt count – 75,000/cmm). Surgery was planned under general anesthesia with bilateral erector spinal block (ESB). The block was administered before the induction and intubation. The left internal jugular vein and right radial artery were cannulated for fluid resuscitation and monitoring. Maintenance anesthesia was done with air, oxygen, and sevoflurane. Intravenous injection morphine was administered to supplement the analgesic effects of ESB. A volume controlled ventilation mode was set with intermittent recruitment maneuver. Intraoperative, the peritoneum and omentum were studded with the tumor deposits [Figure 1] along with tumor deposits over the surface of the urinary bladder, spleen, undersurface of the diaphragm, pancreas, and sigmoid colon. Since optimal cytoreduction is paramount for the outcome and survival, the patient underwent an extensive cytoreductive surgery. The cytoreduction involved resection of primary carcinomatous nodules, appendectomy, cholecystectomy, splenectomy, distal pancreatectomy, sigmoid colectomy, omentectomy, and stripping of diaphragm [Figure 2]. Surgery took about 7 h followed by intraperitoneal chemotherapy with a specialized performer HT machine [Figure 3] by an open coliseum technique [Figure 4]. Prehydration was done with 500 ml normal saline along with inj. ranitidine 50 mg and injection dexamethasone and fosaprepitant were administered to prevent chemotherapy-induced emesis The intraperitoneal chemotherapy was cisplatin 50 mg/m2 and doxorubicin 20 mg/m2 dissolved in 3.5 L of 1.5% dextrose (dialysis fluid) at a temperature of 43°C with a flow rate of 1 L/min with a total hold time of 90 min. The patient had 4800 ml blood loss (maximum allowable blood loss of 1050 ml); we transfused six PRBC, four FFP, and two SDP units. Blood pressure was managed with vasopressors (noradrenaline and vasopressin) infusion. Metabolic acidosis was expected in the patient; it was managed with optimal fluid resuscitation and hourly arterial blood gas monitoring. The patient temperature was managed between 37°C and 39°C. the patient had urine output >0.5 ml/Kg/h. During HIPEC, we administered furosemide, low-dose dopamine infusion, and mannitol to maintain urine output >2 ml/Kg/h. Post chemotherapy the patient received parenteral magnesium sulphate and potassium chloride to prevent cisplatin induced hypomagnesemia. The patient was shifted to ICU for elective ventilation. He was extubated after 2 days and shifted to ward. The patient had anastomotic leak on day 5 for which he underwent colostomy and later colostomy closure. The surgical specimen obtained [Figure 2] was sent for histopathology in separate bottles along with a comprehensive somatic cancer panel. The comprehensive cancer pane was reported as PPC with ER/WT1/CK 7 positive and MSI-High (MMR deficient). Post-operative radiological imaging was suggestive of complete resolution of all initial lesions (complete response). The patient was kept on follow-up with periodic hemogram, CA125 levels, and USG abdomen with CECT in case of any evidence of relapse.

- The omentum and the peritoneum studded with the tumor deposits as seen intraoperatively.

- The post-operative surgical specimen from various sites.

- Performer HT specialized machine for HIPEC with its inlet/outlet tubings and chemotherapy bag.

- (a) The abdominal wall is lifted to prepare a cavity to accept 3–4 L chemotherapy solution. (b) Coliseum technique of hyperthermic intraperitoneal chemotherapy showing abdominal cavity covering with thin plastic sheet during the chemotherapy holding period.
DISCUSSION
PPC diffusely involves the peritoneum in the absence of an obvious primary site. The age-adjusted incidence rate is described as 6.78 per million.[3] Most reported cases of PPC have been described in women, usually elderly while rarely; cases are reported in children and young males. The median survival time is 23.5 months and 5-year survival rates range from 0% to 26.5%.[4]
Once the diagnosis was established, maximal cytoreduction becomes the primary goal of the management.[3] HIPEC involves a procedure of peritoneal lavage by chemotherapeutic agents at a higher temperature. It delivers the cytotoxic drugs into the peritoneal cavity to have a regional dose intensification. Additional hyperthermia potentiates the cytotoxic effects and enhances the tissue penetration.[2] The anesthetic goals of this surgical procedure are to maintain normovolemia, normothermia, avoiding coagulopathy, maintaining urine output, correcting metabolic derangements during such extensive and prolonged surgery, and a smooth post-operative recovery.[5,6]
Blood loss was predicted in such extensive surgeries. Damage control resuscitation protocol is the standard recommendation in many centers for similar procedures.[7,8] Hence, we arranged adequate type specific blood products preoperatively; before incision central venous line and arterial line were established to continuously monitor intravascular fluid status. Injection noradrenaline was started early in the resuscitation which was supported with fluids and blood product transfusion to prevent hypotension [Figure 5].
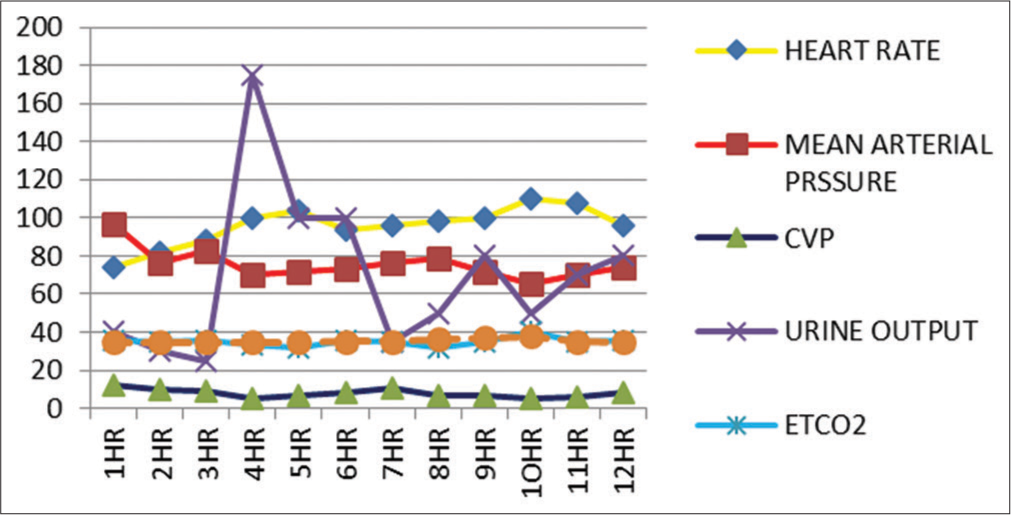
- Chart showing variations in different parameters during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.
After the CRS, chemoperfusion is carried out by an open or closed abdomen technique.[2] We followed the open coliseum technique, where the incised ends of abdominal wall were made to hang with the help of sutures to form a cavity of 3–4 L capacity [Figure 4a]. A Tenckhoff catheter and four drains were placed through the abdominal wall and were fixed watertight with purse string sutures. A Performer HT specialized machine was used to circulate the chemotherapy solution at a set temperature (43°C). The machine was used to pump the fluid into the abdominal cavity through Tenckhoff catheter at a rate of 1 L/min and received the drainage fluid through another two drainage ports. Holding time of 90 min was set to achieve optimal contact of chemotherapeutic agent (cisplatin and doxorubicin). The loss of heat from the open abdomen was minimized by covering the cavity with thin plastic sheet [Figure 4b].
Since these chemotherapeutic agents are nephrotoxic, it is recommended to maintain urine output approximately 2–4 ml/Kg/h during HIPEC.[5] The post-operative samples obtained should be sent in different bottles for HPE analysis. Somatic cancer panel was sent in our case to detect targetable driver/pathogenic mutations as the patient was a male and therefore treating on lines of ovarian cancer by extrapolation as adjuvant or in case of relapse is not the correct approach due to likely different biology of PPC. Patient was detected to be MSI-High. In case of relapse/recurrence Pembrolizumab is an option for this patient.[9]
Certain general safety measures are recommended while HIPEC is used to avoid the adverse effect of hazardous drug exposure.[10] Studies showed that during HIPEC, there is an increase in metabolic rate, oxygen consumption, cardiac output, and heart rate, ETCO2 with metabolic acidosis.[10] We observed all these hemodynamic alterations and managed conservatively. We electively ventilated the patient for nearly 48 h and extubated when all vasopressors were stopped.
Post-operative pulmonary complication is a major concern in a prolonged abdominal surgery. The patient had risk factors such as prolonged surgery, upper abdominal incision, and blood transfusion.[11,12] Adequate pain relief along with lung protective ventilation strategy prevents such pulmonary complications.[12]
CONCLUSION
Cytoreduction with HIPEC is the standard of care in the therapy of peritoneal surface malignancies.[1,2] These extensive yet optimal surgeries are the backbone of a good outcome. Downsizing the tumor with neoadjuvant chemotherapy and then augmenting this with HIPEC are the ideal treatment in PPC. These cases due to the extensive disease create challenges due to full large abdominal mass, major fluid shifts, massive blood loss, and risk of nephropathy, predicted temperature variation, and possible electrolyte imbalance. Post-operative period also is expected to be stormy due to complicated surgery, recovery from blood loss in the settings of chemotherapy. Hence, a closed group management with oncosurgeon, medical oncologist, anesthesiologist, intensivist, and nursing team is required to handle such complicated cases. Newer targetable driver mutations are being discovered and provide suitable treatment strategies in these patients, therefore, tissue testing in these cases is recommended.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Extraovarian primary peritoneal carcinomatosis: A Case Report. Am J Case Rep. 2017;18:714-8.
- [CrossRef] [Google Scholar]
- Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol. 2010;2:68-75.
- [CrossRef] [Google Scholar]
- Results of interval debulking surgery compared with primary debulking surgery in advanced stage ovarian cancer. J Am Coll Surg. 2003;197:955-63.
- [CrossRef] [Google Scholar]
- Primary peritoneal serous carcinoma, an extremely rare malignancy: A case report and review of the literature. Oncol Lett. 2016;11:4063-5.
- [CrossRef] [Google Scholar]
- Anaesthetic implications in hyperthermic intraperitoneal chemotherapy. J Anaesthesiol Clin Pharmacol. 2019;35:3-11.
- [CrossRef] [Google Scholar]
- Anaesthetic management of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: The importance of hydro-electrolytic and acid-base control. Int J Surg Case Rep. 2017;38:1-4.
- [CrossRef] [Google Scholar]
- Damage control resuscitation: History, theory and technique. Can J Surg. 2014;57:55-60.
- [CrossRef] [Google Scholar]
- Damage control resuscitation: A practical approach for severely hemorrhagic patients and its effects on trauma surgery. Mizobata J Intensive Care. 2017;5:4.
- [CrossRef] [Google Scholar]
- FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753-8.
- [CrossRef] [Google Scholar]
- Anaesthesia for Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. Anaesthesia Tutorial of the Week #379.
- [Google Scholar]
- Postoperative pulmonary complications after non-cardiothoracic surgery. Indian J Anaesth. 2015;59:599-605.
- [CrossRef] [Google Scholar]






