Translate this page into:
The impact of myeloma induction therapy on stem cell mobilization and collection: ‘Daratumumab,’ a boon or a bane?

*Corresponding author: Faheema Hasan, Assistant Professor, Department of Hematology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India. 1faheemahasan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mamlekar HH, Hasan F, Yadav S, Kashyap R, Rahman K, Gupta R, et al. The impact of myeloma induction therapy on stem cell mobilization and collection : ‘Daratumumab,’ a boon or a bane? Int J Mol Immuno Oncol. 2024;9:111-5. doi: 10.25259/IJMIO_16_2024
Abstract
Objectives:
Multiple Myeloma (MM) therapy has evolved over many years. While first-line induction therapy has undergone drastic changes, Autologous hematopoietic stem cell transplantation (ASCT) still plays a significant role in consolidation, improving both the depth of response and progression-free survival. Our study aimed at assessing the impact of myeloma induction therapy on stem cell mobilisation and collection.
Material and Methods:
In our study, data of 29 Myeloma patients who underwent ASCT at SGPGI, Lucknow from 2014 to 2023 was retrospectively analyzed. Patients were stratified into cohorts according to the induction therapy received prior to transplant. Outcomes were compared among cohorts who received prior therapy with Immunomodulatory drugs (Lenalidomide, Pomalidomide) (n=18), Proteosome inhibitors (Bortezomib, Carfilzomib) (n=29) and Monoclonal antibody (Daratumumab) based regimen (n= 5). Also, the dose of stem cell infused was correlated with the day of engraftment of stem cells. The number of induction cycles received prior to transplant were correlated with the use of Plerixafor for mobilization of stem cells.
Results:
A greater number of stem cell yield was reported in the group who received Immunomodulatory drugs and Proteosome Inhibitors as compared to Daratumumab based therapy. Prior induction regimen with Immunomodulatory drugs did not significantly alter stem cell mobilization (p= 0.707). However, Daratumumab based regimen showed significantly lower yield of stem cells (p <0.001). No significant correlation was noticed between the average number of prior induction cycles and the use of Plerixafor for mobilization of stem cells (p= 0.551). No significant correlation was noticed between the dose of stem cells and the day of engraftment (p= 0.094).
Conclusion:
Albeit small numbers, this study highlights the fact that treatment with Daratumumab especially in a relapsed, refractory setting impairs stem cell mobilization and warrants larger studies for further correlation.
Keywords
Multiple myeloma
Induction
Hematopoietic stem cell mobilization
Hematopoietic stem cell transplantation
INTRODUCTION
Multiple myeloma (MM) therapy has evolved over many years, with a wide range of therapies being available in the current era. With the arrival of novel therapies such as immunomodulatory agents, proteasome inhibitors, and monoclonal antibodies such as daratumumab, there has been an unprecedented level of deeper hematologic responses that have been observed.
However, despite the positive impact of newer agents on response and survival, MM remains incurable and standard care still incorporates a consolidative autologous stem cell transplant for deeper response and better progression-free survival.[1-4]
The collection of adequate numbers of hematopoietic stem cells (CD 34+ cells) is necessary for rapid and sustained engraftment following allogeneic hematopoietic stem cell transplantation (aHSCT). A minimum of 2.0 × 106 CD34+ cells/kg is needed for adequate bone marrow function after autologous hematopoietic stem cell transplantation (ASCT).[5]
Most myeloma patients eventually relapse, and hence, a second tandem aHSCT is a potential treatment option, and the collection of adequate CD34+ cells for two transplants is routinely performed.[6]
Since prior induction regimens can result in myelotoxicity, it is important to recognize their effect on hematopoietic stem and progenitor cell (HSPC) mobilization and collection outcomes. Granulocyte colony-stimulating factor alone or in combination with cyclophosphamide or plerixafor is used in the mobilization of CD34+ cells.[7-9]
Keeping into consideration with the changing landscape of induction therapy, we report our experience in a cohort of patients with myeloma and compare the impact of myeloma induction regimens used upon the subsequent efficiency of HSPC mobilization and collection.
Aims and objectives
To study the impact of myeloma induction therapy on hematopoietic stem cell mobilization and collection
To study the correlation between stem cell dose infused upon the day of engraftment
To study the correlation between average induction cycles and the use of plerixafor for stem cell mobilization.
MATERIAL AND METHODS
Study design
This is a single-centre, retrospective study in Northern India.
Data regarding the mobilization of hematopoietic stem cells of 29 myeloma patients who underwent autologous stem cell transplant at Sanjay Gandhi Postgraduate Institute, Lucknow, from 2014 to 2023 were retrospectively reviewed.
The data were retrospectively extracted from hospital electronic medical records. Information collected included patients’ demographic data and their disease description comprising baseline characteristics, induction therapy received prior, dose of stem cells mobilized, their day of engraftment, and whether plerixafor was used for mobilization of stem cells or not.
Patients were stratified into cohorts according to the induction therapy received before the transplant. Outcomes were compared among cohorts who received prior therapy with immunomodulatory drugs (lenalidomide and pomalidomide) (n = 18), proteasome inhibitors (bortezomib and carfilzomib) (n = 29), and monoclonal antibody (daratumumab)-based regimen (n = 5).
Furthermore, the dose of stem cells infused during ASCT was correlated with the day of engraftment of stem cells. The number of induction cycles received before transplant was correlated with the use of plerixafor for mobilization of stem cells.
Data were analyzed in the Statistical Package for the Social Sciences software using appropriate statistical tests.
RESULTS
Demographic details
The demographic details of the study population has been shown in Table 1.
| Demographic Parameters | Observation |
|---|---|
| Total myeloma transplant cohort | 29 |
| Median age | 50 years (44–67) |
| Males | 68.9% (n=20) |
| Females | 31.03% (n=09) |
Revised Multiple Myeloma International staging system (R-ISS) staging was available for 20 patients, and 14 out of 20 (70%) had stage III disease at diagnosis. High-risk cytogenetics were identified in 16 patients (55%). Six patients (37%) had t (4; 14), 11 had gain 1q (68%), 5 had del1p32 (31.25%), and 4 were TP53 mutated (25%).
A greater number of stem cell yields was reported in the group who received immunomodulatory drugs and proteasome inhibitors as compared to daratumumab-based therapy. Prior induction regimens with immunomodulatory drugs did not significantly alter stem cell mobilization (P = 0.937). The type of induction therapy used prior to ASCT and the dose of stem cells harvested has been shown in Table 2. Figure 1 shows the correlation between the use of immunomodulatory drugs in induction and the dose of stem cells harvested.
| Dose of stem cells (106 cells/kg) | P-value | |||
|---|---|---|---|---|
| Prior induction therapy | Mean | Median | Percentile (P25–P75) | |
| Immunomodulatory drugs | ||||
| Not received (n=11) | 5.0±1.0 | 5.3 | 5–5.5 | 0.707 |
| Received (n=18) | 4.9±1.2 | 5.0 | 4.5–5.9 | |
| Proteasome inhibitors | ||||
| Not Received (n=0) | -- | -- | -- | -- |
| Received (n=29) | 5.0±1.11 | 5 | 4.6–5.6 | |
| Monoclonal antibody (daratumumab)-based therapy | ||||
| Not received (n=24) | 5.4±0.73 | 5.4 | 4.9–5.9 | <0.001 |
| Received (n=5) | 3.1±0.70 | 3 | 2.7–3.1 | |
ASCT: Autologous hematopoietic stem cell transplantation
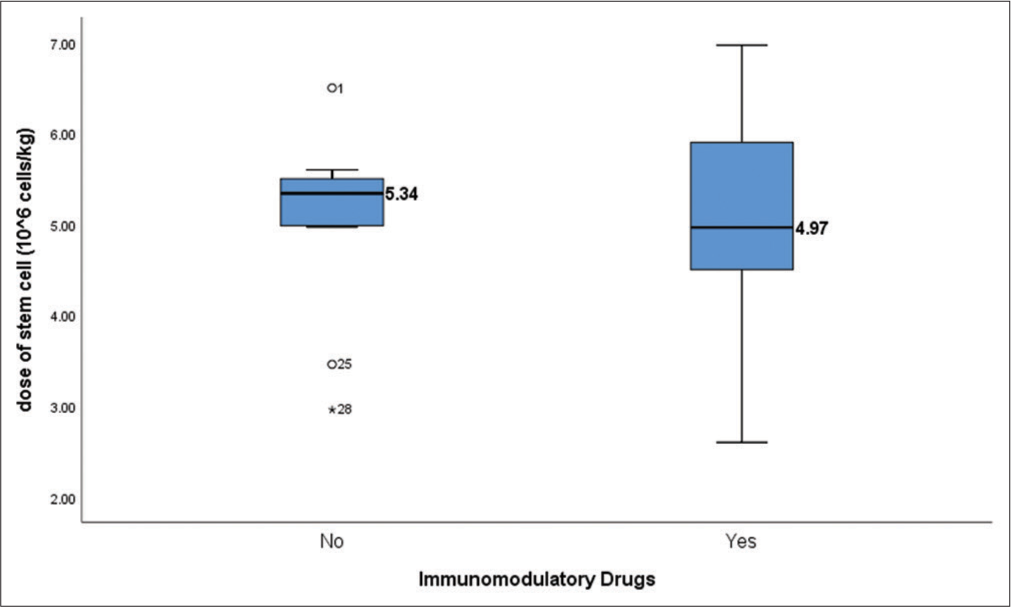
- Box plot showing correlation between the use of immunomodulatory drugs and dose of stem cell mobilized (P = 0.707). Immunomodulatory drugs include lenalidomide and Pomalidomide. Dose of stem cells harvested is mentioned in Million cells/kg.
All 5 patients who received daratumumab as their induction therapy had difficulty in mobilization and needed plerixafor on day 4. Daratumumab-based regimen showed significantly lower yield of CD34+ stem cells (P ≤ 0.001). Table 3 shows the use of Plerixafor for stem cell mobilisation prior to ASCT and the average induction cycles received prior to ASCT.
Box plot depicting the correlation between the use of Daratumumab during induction and the dose of stem cell harvested is shown in Figure 2.
| Parameters | Observation |
|---|---|
| Patients who received plerixafor for mobilization on day 4 | 19/29 (65.51%) |
| Average number of induction cycles in Plerixafor group | 10 (Range: 6–26) |
| Average number of induction cycles in non-plerixafor group | 7 (Range: 4–12) |
| Average day of engraftment in plerixafor group | 12 (Range: 8–27) |
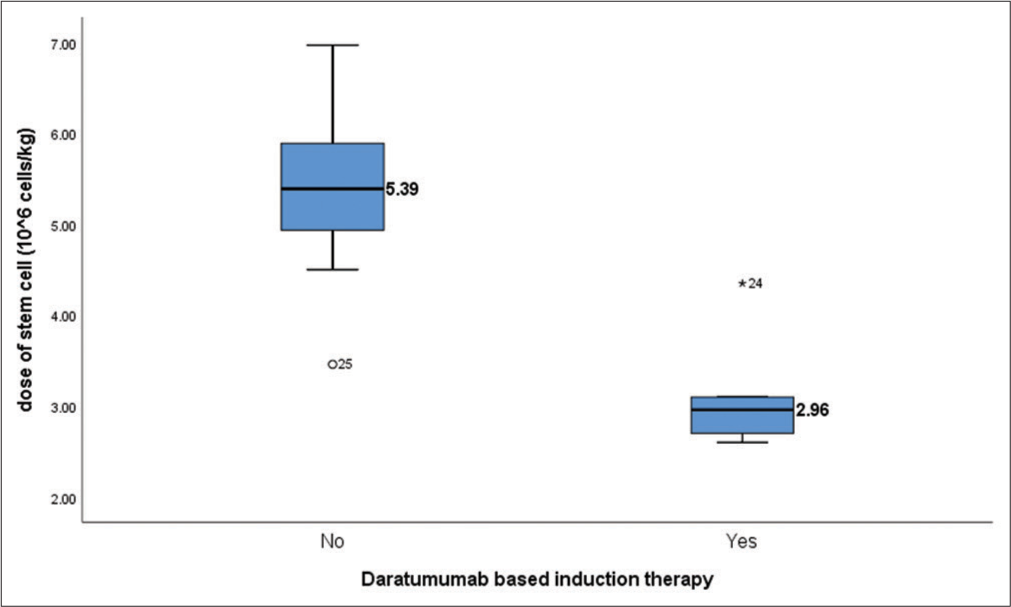
- Box plot showing correlation between the use of daratumumab and dose of stem cell mobilized (P < 0.001). Dose of stem cells harvested are mentioned in Million cells/kg.
No significant correlation was noticed between the dose of stem cells and the day of engraftment (P = 0.094). Scatter plot depicting the correlation between the dose of stem cells infused and the day of engraftment is shown in Figure 3.
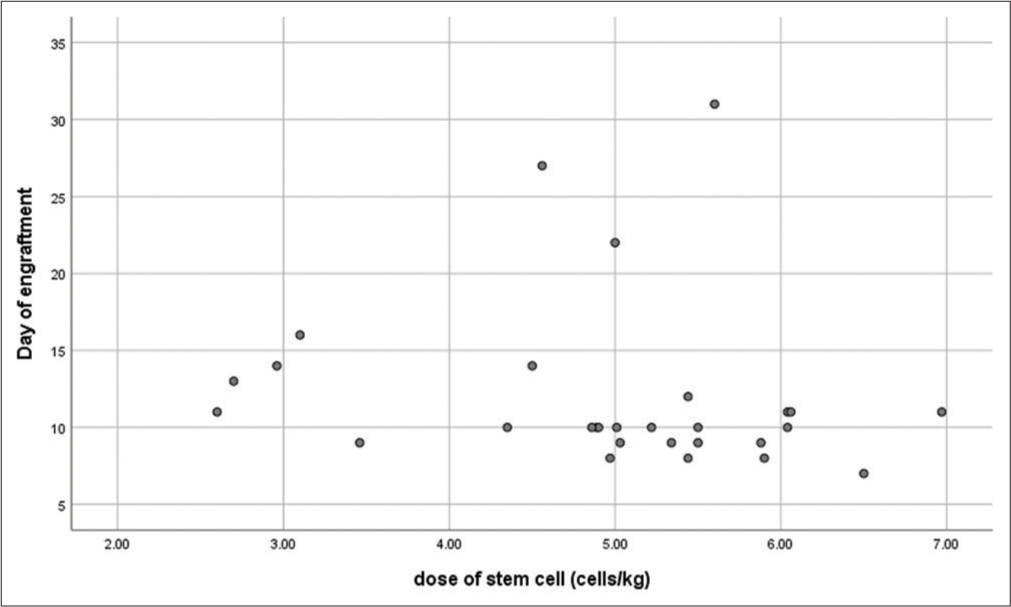
- Scatter plot depicting the correlation between the dose of stem cells infused and the day of engraftment. The dose of stem cells harvested is mentioned in Million cells/kg. The day of engraftment refers to the day of neutrophil engraftment. (r = 0.317, P = 0.094).
Scatter plot depicting the correlation between dose of stem cells harvested and the average induction cycles prior to ASCT is shown in Figure 4.
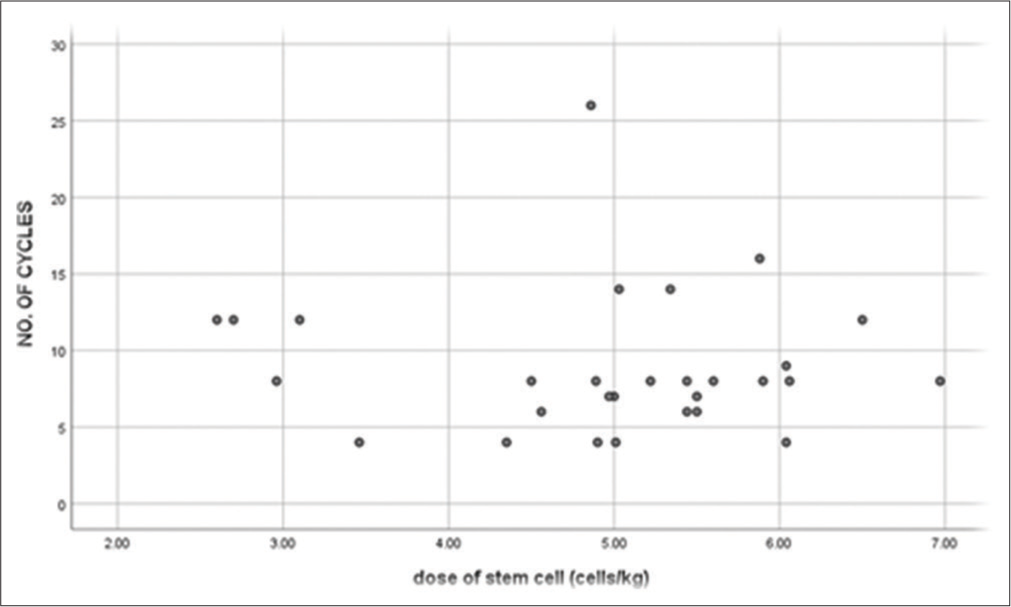
- Scatter plot depicting the correlation between the dose of stem cells harvested and the total number of induction cycles received prior to transplant. The dose of stem cells harvested is mentioned in Million cells/kg. Induction therapy prior to transplant include a triplet therapy incorporation a Proteosome inhibitor, immunomodulator and steroid. Quadruplet therapy in addition contains a monoclonal antibody(Daratumumab). Doublet induction incorporates a Proteosome inhibitor or immunomodulator and steroid (r = −0.029, P = 0.880).
No significant correlation was noticed between the average number of prior induction cycles and the use of plerixafor for mobilization of stem cells (P = 0.551). Box plot depicting the comparison between use of plerixafor and the average induction cycles received prior to ASCT is shown in Figure 5.
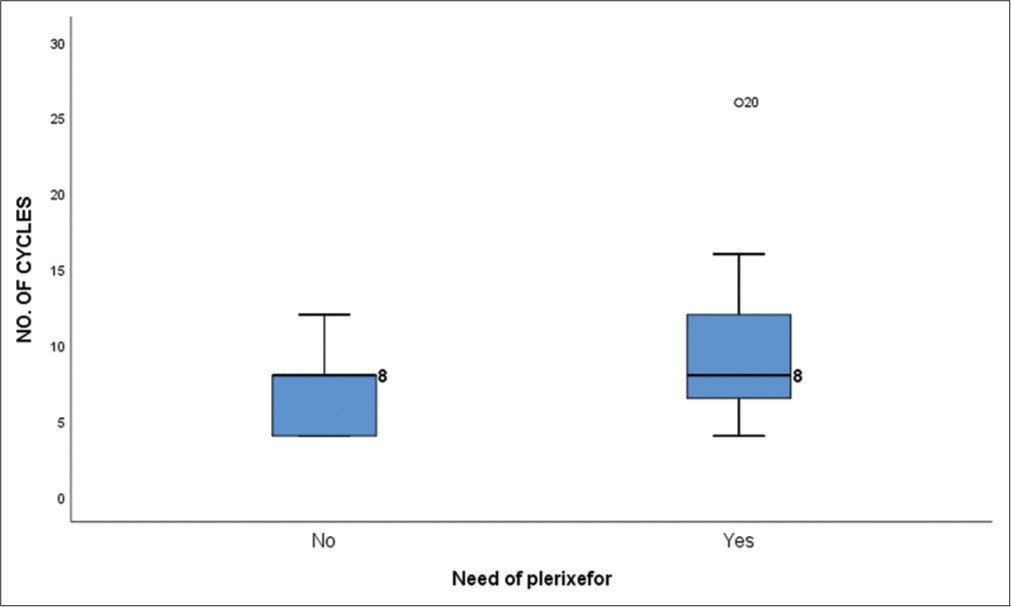
- Box plot showing the comparison between the use of plerixafor for stem cell mobilization and the average number of induction cycles received prior to transplant (P = 0.330).
Figure 6 shows a bar graph depicting the impact of Daratumumab based induction therapy on the dose of stem cells harvested prior to ASCT.
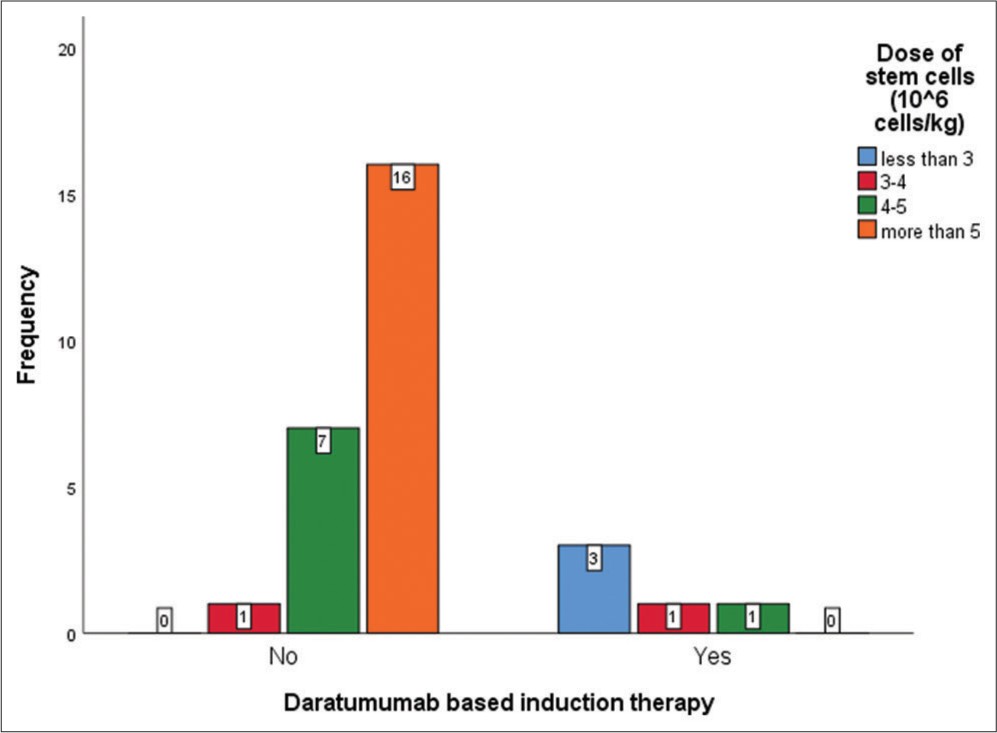
- Bar graph showing the impact of Daratumumab based induction therapy on the dose of stem cells harvested prior to transplant. X-Axis depicts whether or not Daratumumab was used during induction and Y-axis depicts the frequency of events.
DISCUSSION
This study describes the impact of prior induction therapy in myeloma patients on hematopoietic stem cell mobilization and collection before ASCT in a single tertiary care center. It also provides data on whether the dose of stem cells mobilized before ASCT can influence the overall outcome, including early engraftment.
We report a similar experience in comparison to other studies suggesting that induction regimens differentially influence stem cell collection. Bortezomib-containing induction therapy is not associated with a detrimental impact on HSPC collection.[10,11] Silvennoinen et al. found that lenalidomide produced negative effects on CD34+ cell mobilization, as the number of CD34+ cells collected by those who received lenalidomide, bortezomib, and dexamethasone was approximately 32% lower than BD-treated patients.[12,13]
Pozotrigo et al. performed a multivariate analysis of a retrospective cohort of 317 patients and found that none of the induction agents, including thalidomide, lenalidomide, bortezomib, and others, were significantly associated with poor HSPC mobilization and collection.[14]
Lemonakis et al. performed a multivariate analysis of 217 myeloma patients and showed that the addition of daratumumab in induction can induce deeper responses but may have a negative effect on stem cell mobilization and collection, leading to lower stem cell yield.[15] It was also associated with increased rescue use of plerixafor.
Similarly, we also found that both of our patients who received daratumumab-based therapy had poor stem cell mobilization and warranted the need for plerixafor. Two out of the 5 patients had just 4 cycles of daratumumab-based induction and still necessitated the use of plerixafor, which highlights the fact that daratumumab, being an anti-CD38 antibody, can cause deficiency of bone marrow stem cells as these also express CD38 to an extent.
Previously identified risk factors for poor stem cell mobilization, such as prior therapy with immunomodulators or proteasome inhibitors, were not associated with lower stem cell yield in our study.
CONCLUSION
We observed that the addition of daratumumab in the myeloma induction regimen significantly impairs stem cell mobilization and collection, leading to poor stem cell yield in both upfront and relapsed settings.
As daratumumab-based quadruplets are rapidly becoming the standard of care in induction, new strategies to facilitate the collection of stem cells should be explored, such as omitting cyclophosphamide in mobilization and monitoring closely and early use of plerixafor in case of low CD34 + cells count in the peripheral blood.
The choice of induction therapy in myeloma patients can alter the results of downstream procedures such as stem cell mobilization and collection.
It is important that ongoing quality initiatives should be put in place to monitor these outcomes to ensure that changes in clinical operations remain beneficial to patients.
Ethical approval
Institutional Review Board approval is not required as it is a retrospective study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Blood graft cellular composition and posttransplant outcomes in myeloma patients mobilized with or without low-dose cyclophosphamide: A randomized comparison. Transfusion. 2016;56:1394-401.
- [CrossRef] [PubMed] [Google Scholar]
- Improving stem cell mobilization strategies: Future directions. Bone Marrow Transplant. 2009;43:181-95.
- [CrossRef] [PubMed] [Google Scholar]
- Novel agent induction therapy alone or followed by autologous stem cell transplantation in younger patients with multiple myeloma: A single-center retrospective study of 114 cases. Mol Clin Oncol. 2016;4:107-13.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: A position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49:865-72.
- [CrossRef] [PubMed] [Google Scholar]
- Optimizing autologous stem cell mobilization strategies to improve patient outcomes: Consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20:295-308.
- [CrossRef] [PubMed] [Google Scholar]
- Hematopoietic stem cell transplantation for multiple myeloma: Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21:1155-66.
- [CrossRef] [PubMed] [Google Scholar]
- Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cost and clinical analysis of autologous hematopoietic stem cell mobilization with G-CSF and plerixafor compared to G-CSF and cyclophosphamide. Biol Blood Marrow Transplant. 2011;17:729-36.
- [CrossRef] [PubMed] [Google Scholar]
- Plerixafor is superior to conventional chemotherapy for first-line stem cell mobilisation, and is effective even in heavily pretreated patients. Blood Cancer J. 2014;4:e255.
- [CrossRef] [PubMed] [Google Scholar]
- Bortezomib in multiple myeloma: Systematic review and clinical considerations. Curr Oncol. 2014;21:e573-603.
- [CrossRef] [PubMed] [Google Scholar]
- Predicting poor peripheral blood stem cell collection in patients with multiple myeloma receiving pre-transplant induction therapy with novel agents and mobilized with cyclophosphamide plus granulocyte-colony stimulating factor: Results from a Gruppo Italiano Malattie EMatologiche dell'Adulto Multiple Myeloma Working Party study. Stem Cell Res Ther. 2015;6:64.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of molecular remission rate after bortezomib plus dexamethasone induction treatment and autologous stem cell transplantation in newly diagnosed multiple myeloma patients. Br J Haematol. 2013;160:561-4.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized phase II study of stem cell mobilization with cyclophosphamide + G-CSF or G-CSF alone after lenalidomide-based induction in multiple myeloma. Bone Marrow Transplant. 2016;51:372-6.
- [CrossRef] [PubMed] [Google Scholar]
- Factors impacting stem cell mobilization failure rate and efficiency in multiple myeloma in the era of novel therapies: Experience at Memorial Sloan Kettering Cancer Center. Bone Marrow Transplant. 2013;48:1033-9.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of daratumumab-based induction on stem cell collection parameters in Swedish myeloma patients. Haematologica. 2023;108:610-4.
- [CrossRef] [PubMed] [Google Scholar]







