Translate this page into:
Long-term efficacy of osimertinib in de novo exon 19 deletion with T790M in an epidermal growth factor receptor mutated lung cancer patient in India

*Corresponding author: Imran Nisar Shaikh, Centre for Cancer, Kokilaben Dhirubhai Ambani Hospital and Medical Research Institute, Mumbai, Maharashtra, India. imran.shaikh@kokilabenhospitals.com
-
Received: ,
Accepted: ,
How to cite this article: Fatima S, Shaikh IN, Mistry R, Kaler AK. Long-term efficacy of osimertinib in de novo exon 19 deletion with T790M in an epidermal growth factor receptor mutated lung cancer patient in India. Int J Mol Immuno Oncol. 2024;9:25-8. doi: 10.25259/IJMIO_16_2023
Abstract
Primary epidermal growth factor receptor (EGFR) T790M mutation is infrequently identified in previously untreated non-small cell lung cancer (NSCLC) patients. There is variation in the frequency of de novo T790M mutations depending on the population examined and the technology used for mutation detection. According to direct sequencing, 0.4–3% of all NSCLCs and 1–8% of all EGFR-mutant NSCLCs show primary T790M mutation. This mutation always coexists with a sensitizing EGFR mutation and is more commonly present along with exon 21 L858R mutation and infrequently with exon 19 deletion. Osimertinib, an oral irreversible third-generation EGFR tyrosine kinase inhibitor, is selective for both, EGFR sensitizing mutations and T790M resistance mutation. AURA trial has confirmed the effectiveness of osimertinib in patients with T790M mutation. The median progression-free survival is better in primary T790M as compared to the acquired mutation in a previously treated NSCLC. The overall survival with osimertinib is still 38.6 months only. Even after good response in both primary and acquired T790M mutation, there is disease progression and shifting to chemotherapy is required. We present an interesting case of a 60-year-old non-smoker female deriving benefit from a single agent osimertinib with a rare combination of mutation at presentation even after 6 years of initiation of therapy.
Keywords
Epidermal growth factor receptor mutation
Epidermal growth factor receptor tyrosine kinase inhibitors
Adenocarcinoma lung
Durable response
INTRODUCTION
Around 85% of lung cancer cases are non-small cell lung cancer (NSCLC). Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI) have revolutionized the treatment of EGFR mutant NSCLC, and all three generations of EGFR are widely used in the treatment.
Based on the results of the Soria et al.[1] study in October 2017, osimertinib is recommended as a first-line treatment for patients with advanced NSCLC with EGFR mutations and was listed as a category I recommendation by the National Comprehensive Cancer Network guidelines in 2019.
Osimertinib is effective against both exon 20 resistant T790M and EGFR sensitizing mutation. The occurrence of primary T790M ranges between 1% and 38%[2] and is commonly associated with exon 21 L858R mutation and less frequently with exon 19 deletion.[3] However, disease progression occurs even with osimertinib with progression-free survival (PFS) of 18.9 months only.[1]
We present an interesting case of a 60-year-old non-smoker female deriving benefit from a single agent third-generation EGFR-TKI with a rare combination of mutation at presentation, even after 6 years of initiation of therapy.
CASE REPORT
A 60-year-old hypertensive female reported to us in November 2016, with complaints of persistent cough and right-sided neck swelling for 1 year. All routine investigations were done which were within normal limits.
On physical examination, no pallor, icterus, or organomegaly were noted but the right-sided level IB cervical and the right supraclavicular lymph nodes were palpable.
A right supraclavicular lymph node wedge biopsy was done. The biopsy revealed metastatic adenocarcinoma with lung primary as the cells showed positivity for thyroid transcription factor 1 (TTF-1) and Napsin A. This confirmed the diagnosis of metastatic adenocarcinoma lung. The tissue sample was also sent for molecular studies.
To evaluate the extent of the disease, a positron emission tomography and computed tomography (PET-CT) scan was done. The PET-CT scan showed a heterogeneously enhancing soft-tissue mass with spiculated margins in the apico-posterior segment of the right upper lobe, measuring 4.1 × 3.5 cm, abutting the overlying costal and mediastinal pleura. Multiple enlarged the right supraclavicular, bilateral high paratracheal, right pre- and para-tracheal, left paraaortic, pre-carinal, subcarinal, and common iliac lymph nodes showed an increase in metabolic activity [Figure 1a].

- (a) A heterogeneously enhancing soft-tissue mass in the apico-posterior segment of the right upper lobe, measuring 4.1 × 3.5 cm along with multiple enlarged surrounding lymph nodes. (b) Reduction in the size of the primary and the lymph nodes post chemotherapy.
Surgery was ruled out due to the advanced nature of the disease. Pending the results of the molecular studies, the patient was started on an injection of pemetrexed and carboplatin once every 21 days.
The interim PET-CT done post 4 cycles of chemotherapy showed a good response with a reduction in size and activity at the primary site and the multiple involved lymph nodes [Figure 1b]. The results of molecular studies were ready by this time. There was a mutation seen in exon 19 (c.2239_2250 del) along with exon 20 (c.2369C > T), also called T790M mutation. The patient was advised tablet osimertinib based on the above results. Due to the unavailability of osimertinib in India at that time, she was continued on pemetrexed and carboplatin.
Post-completion of 6 cycles of pemetrexed and carboplatin, the PET-CT scan continued to show a good response. Thus, the patient was started on maintenance pemetrexed every 21 days. Post 6 cycles of maintenance, the PET-CT scan showed an increase in the activity of the submandibular and the right supraclavicular lymph nodes suggestive of progressive disease [Figure 2a and b]. By this time, osimertinib was available in India. Thus, the patient was started on the tablet osimertinib 80 mg once a day in July 2017. The PET-CT scan done post 3 months with osimertinib showed a reduction in size and activity of submandibular and the right supraclavicular lymph nodes.
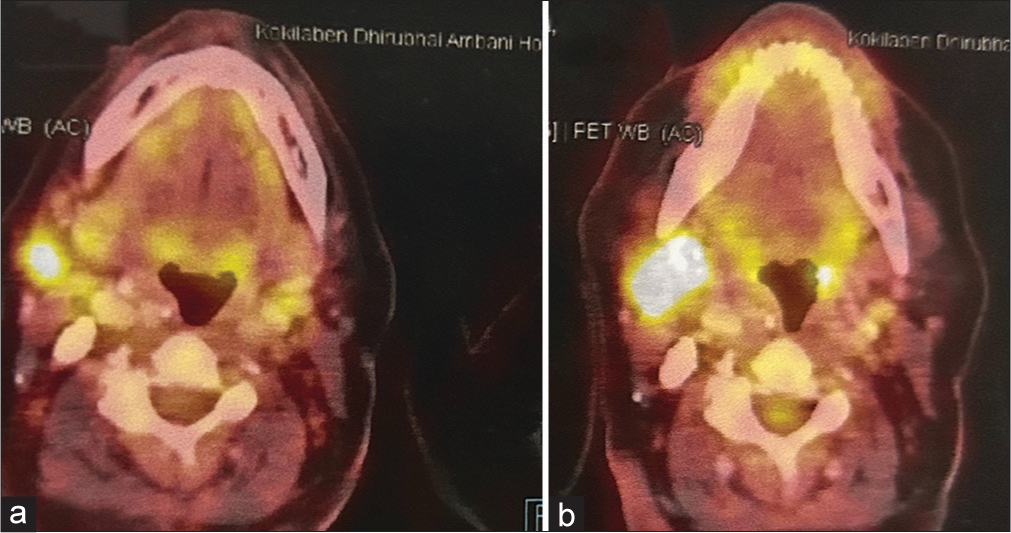
- (a) Reduction in the activity and size of the submandibular lymph node post 6 cycles of pemetrexed and carboplatin. (b) Increase in the activity and size of the submandibular lymph node post 4 cycles of maintenance pemetrexed.
She continued on tablet osimertinib from July 2017 to date with only slight darkening of the skin of the face and the hand with no other toxicities. She has been on regular follow-ups since 2017 and has had no relapse or intolerable toxicities in these 6 years.
DISCUSSION
The incidence of EGFR (exons 18–21) mutation in the Indian population is 23%. EGFR exon 19 deletion and exon 21 mutation together constitute >90% of the EGFR-positive cases.[4] There is variation in the incidence of primary T790M NSCLC ranging between 1% and 38% and it depends on the detection method applied and the study population.[2] In a study conducted by Panda et al., the incidence is 2.59% in the Indian population.[5]
In a study conducted by Wang et al., the frequency of primary T790M with exon 19 deletion was 11% as compared to L858R (45%). Our patient had co-existence of primary T790M with exon 19 deletion.[6]
Osimertinib is an oral, third-generation EGFR-TKI inhibitor, that has an excellent inhibitory effect on the T790 M mutation, especially with the central nervous system metastasis.[6] Patients with NSCLC who have progressed to T790 M mutation after treatment with EGFR-TKIs, show excellent response to osimertinib.[7] The AURA3 study showed an overall response rate of 71% and PFS of 10.1 months in treating T790 M mutant NSCLC patients compared to chemotherapy.[8] The median overall survival (OS) was 38.6 months with osimertinib in the study conducted by Ramalingam et al.[9]
Meanwhile, for TKI-naive patients with sensitive EGFR mutations, the median PFS of osimertinib was 22.1 months.[10] However, limited data are available regarding the effectiveness of osimertinib in patients with primary T790M mutation. Many case reports have indicated that such patients tend to respond well to osimertinib. The median PFS of 8 months was highlighted for four primary T790M mutant patients who received osimertinib in a study conducted in China.[11] Wang et al. reported patients with primary T790M mutation responded well to osimertinib and had a median PFS of 17.0 months, which was significantly longer in comparison to 10.0 months for patients with acquired T790M mutation. However, the median OS of acquired T790M mutation patients was significantly longer as compared to that of primary T790M mutation patients who received osimertinib.[6]
Osimertinib can affect both T790M and 19del/21L858R positions in primary T790M mutant patients. Thus, this high affinity of osimertinib to both sites of EGFR mutations accounts for a good response to the therapy. However, in patients with acquired T790M mutation, the selection of first TKIs could result in the inhibition or activation of certain signaling pathways in upstream or downstream of EGFR or the upregulated or downregulated expression of certain proteins, which causes differential response to the osimertinib treatment.[12]
Preliminary data suggest that first-line osimertinib resistance mechanisms are similar to those seen in patients with the T790M mutation receiving osimertinib as a second-line therapy. The most common resistance mechanisms include the C797S mutation in exon 20 (7%) and MET amplification (15%). HER2 amplification, BRAF, KRAS, PIK3CA, or rare EGFR secondary mutations, also lead to resistance development. The other mutations include M766Q, S768I, and L718V in exon 20 along with the loss of T790M mutation due to prolonged treatment with osimertinib.[13,14] In the study conducted by Wang et al., there was rapid progression seen in the patient of primary T790M after the development of osimertinib resistance and died after 2–3 cycles of chemotherapy. However, the patient with acquired T790M had multiple therapy options before and after osimertinib.[6]
To conclude, our patient with both exon 19 and exon 20 T790M mutation since presentation was started on tablet osimertinib in 2017. The patient is still continuing to derive the benefit without any relapse, intolerable toxicities, or development of resistance mechanisms. To the best of our knowledge, this is the first longest PFS case from India (non-oriental ethnicity).
CONCLUSION
Our study reiterates the benefit of osimertinib as first-line therapy in primary exon 20 T790M mutations with PFS of around 6 years with limited toxicity and the absence of resistance to the treatment.
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
Patient consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113-25.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366-77.
- [CrossRef] [PubMed] [Google Scholar]
- Coexistence of EGFR T790M mutation and common activating mutations in pretreatment non-small cell lung cancer: A systematic review and meta-analysis. Lung Cancer. 2016;94:46-53.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency of EGFR mutations in 907 lung adenocarcioma patients of Indian ethnicity. PLoS One. 2013;8:e76164.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment pattern and outcomes in de novo T790M-mutated non-small cell lung cancer. Ecancermedicalscience. 2022;16:1385.
- [CrossRef] [PubMed] [Google Scholar]
- Different characteristics and survival in non-small cell lung cancer patients with primary and acquired EGFR T790M mutation. Int J Cancer. 2019;144:2880-6.
- [CrossRef] [PubMed] [Google Scholar]
- Transformation to small-cell lung cancer following treatment with EGFR tyrosine kinase inhibitors in a patient with lung adenocarcinoma. Lung Cancer. 2013;82:370-2.
- [CrossRef] [PubMed] [Google Scholar]
- Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629-40.
- [CrossRef] [PubMed] [Google Scholar]
- Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41-50.
- [CrossRef] [PubMed] [Google Scholar]
- Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:841-9.
- [CrossRef] [PubMed] [Google Scholar]
- Primary and acquired EGFR T790M-mutant NSCLC patients identified by routine mutation testing show different characteristics but may both respond to osimertinib treatment. Cancer Lett. 2018;423:9-15.
- [CrossRef] [PubMed] [Google Scholar]
- Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev. 2018;65:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725-37.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of resistance to osimertinib. J Thorac Dis. 2020;12:2851-8.
- [CrossRef] [PubMed] [Google Scholar]







