Translate this page into:
L1CAM expressing, ZFTA:: NCOA1 fusion-positive supratentorial ependymoma: A case report

*Corresponding author: Roshani Hansraj Gala, Department of Oncopathology, Neuberg Oncopath, Mumbai, Maharashtra, India. roshanigala@yahoo.in
-
Received: ,
Accepted: ,
How to cite this article: Gala RH, Koli K, Mehta J. L1CAM expressing, ZFTA:: NCOA1 fusion-positive supratentorial ependymoma: A case report. Int J Mol Immuno Oncol 2023;8:110-4.
Abstract
Ependymomas are uncommon central nervous system tumors arising from the ependymal lining of the ventricular system. Clinically, ependymomas are a heterogeneous group of tumors ranging from benign subependymomas to very aggressive and often fatal childhood ependymomas of the posterior fossa. The previous edition (2016) of the World Health Organization (WHO) classification primarily defined ependymoma subtypes based on their clinicopathological characteristics (with the exception of v-rel avian reticuloendotheliosis viral oncogene homolog A (RELA) RELA-fusion-positive ependymoma), while the WHO 2021 classification instead classifies ependymoma on the basis of their molecular profile and anatomic location. These include supratentorial-zinc finger translocation-associated (ZFTA) fusion-positive, supratentorial-yes-associated protein 1 (YAP 1) fusion-positive, posterior fossa group A and B, spinal, spinal-master regulator of cell cycle entry and proliferative metabolism (MYCN)-amplified, myxopapillary, and subependymoma subtypes. This new approach provides an objective molecular basis for the diagnosis as well as classification of ependymomas. At the same time, it is also helpful to better predict the prognosis of the patients. Notably, first studies on tumor relapse samples indicate that this molecular classification might be more stable in the course of the disease than histology alone. Among these, ZFTA-fusion-positive supratentorial ependymomas (STEs) have the worst outcome and non-RELA ZFTA-fusion ependymomas have even worse outcome; hence, recognition of this fusion is important. L1 cell adhesion molecule (L1CAM) immunoexpression is specific for ZFTA:: RELA fusion and supports the diagnosis of ZFTA-fusion-positive STE where molecular testing is unavailable. We describe a case of L1CAM immunoexpressing and ZFTA:: NCOA fusion-positive STE.
Keywords
Supratentorial ependymoma
L1 cell adhesion molecule
ZFTA fusion
INTRODUCTION
Ependymomas are uncommon central nervous system (CNS) tumors arising from the ependymal lining of the ventricular system. Clinically, ependymomas are a heterogeneous group of tumors ranging from benign subependymomas to very aggressive and often fatal childhood ependymomas of the posterior fossa.[1] The World Health Organization (WHO) 2021 update of the WHO classification of CNS tumors classifies ependymomas based on molecular profiles and anatomic location, which include supratentorial-zinc finger translocation-associated (ZFTA) fusion-positive, supratentorial-YAP1 fusion-positive, posterior fossa group A and B, spinal, spinal-MYCN-amplified, myxopapillary, and subependymoma subtypes. This not only provides an objective molecular basis for the diagnosis and classification of ependymomas but is also intended to better predict the clinical outcome of the patients.[2,3]
CASE REPORT
A 4-year-old male child presented with a left parieto-occipital space-occupying lesion which was excised and subsequently recurred after 2 months. The magnetic resonance imaging (MRI) of brain revealed a 7 × 5.3 × 5 cm sized intra-axial mass with cystic and solid areas, bright post-contrast enhancement, and perilesional edema [Figure 1]. The recurrent tumor was excised and analyzed.

- Magnetic resonance imaging brain showing left parieto-occipital intra axial mass with cystic and solid areas, bright post-contrast enhancement, and perilesional edema.
Microscopic examination revealed a solid tumor with reasonable circumscription; however, in foci distinct, infiltration into normal brain parenchyma was identified. The tumor revealed scattered hypercellular nodules comprised of round cells with speckled chromatin, inconspicuous nucleoli, and scant cytoplasm [Figure 2a and b]. Hypocellular areas with fibrillary neuropil-like stroma were also present [Figure 2a and c]. Occasional perivascular rosettes were noted [Figure 2d]. Epithelial structures in the form of ependymal tubules, canals, and cribriform glands were identified [Figure 2e]. Occasional areas resembling tanycytic differentiation were seen. Brisk mitotic activity was noted (approximately 10–12/10 hpf) [Figure 2f]. The tumor showed foci of microvascular proliferation [Figure 2g], necrosis [Figure 2h], and scattered foci of dystrophic calcification [Figure 2i].

- Microscopic features. (a) variable cellularity (×5), (b) hypercellular nodules (×20), (c) hypocellular areas with fibrillary neuropil-like stroma (×20), (d) perivascular pseudorosettes (×20), (e) epithelial structures (×20), (f) mitoses (×60), (g) microvascular proliferation (×40), (h) necrosis (×10), and (i) dystrophic calcification (×20).
On immunohistochemistry (IHC), the fibrillary areas of the tumor expressed glial fibrillary acidic protein (GFAP) [Figure 3a], S-100 protein [Figure 3b], and synaptophysin [Figure 3c]. The epithelial structures expressed cytokeratin [Figure 3d], epithelial membrane antigen (EMA) [Figure 3e], oligodendrocyte transcription factor 2 (Olig-2) [Figure 3f], and D240 [Figure 3g]. The ependymal cells in the hypercellular nodules showed dot-like positivity with EMA [Figure 3e] and were immunonegative for synaptophysin, Neu N [Figure 3h], GFAP [Figure 3a], and cytokeratin [Figure 3d]. The mind bomb-1 (Mib-1) labeling index in the epithelial areas was approximately 80%. In the hypercellular nodules, the Mib-1 labeling ranged from 20% to 50% [Figure 3i]. Nuclear integrase interactor 1 (INI-1) expression was retained by the tumor cells. The tumor cells showed membranous immunoexpression of L1 cell adhesion molecule (L1CAM) (IHC for L1CAM was performed at NIMHANS, India) [Figure 4a]. Molecular analysis by next-generation sequencing revealed a ZFTA:: NCOA1 fusion [Figure 4b].

- Immunohistochemistry. (a) Glial fibrillary acidic protein (×10). (b) S-100p (×10), (c) synaptophysin (×20), (d) cytokeratin (×5), (e) epithelial membrane antigen (×20), (f) Oligodendrocyte transcription factor 2 (×10), (g) D240 (×10), (h) Neu N (×10), and (i) Mind bomb-1 (×5).
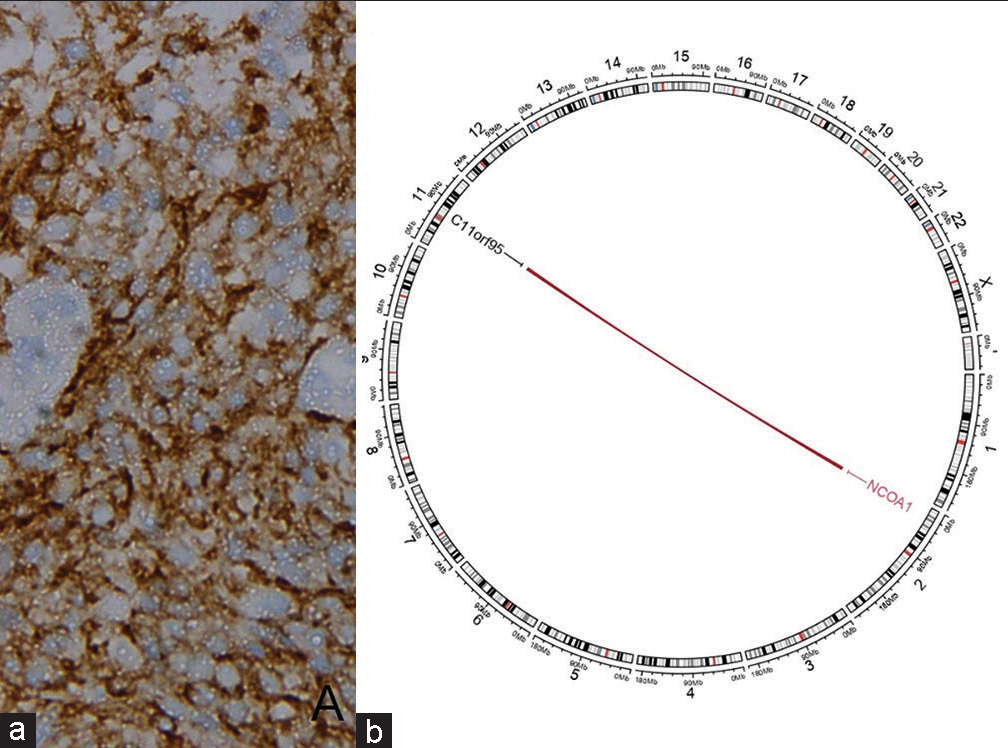
- (a) L1 cell adhesion molecule immunoexpression, (b) Circos plot of next generation sequencing showing ZFTA:: NCOA1 fusion.
In the context of the highly cellular tumor nodules showing brisk mitotic activity, a high Mib-1 labeling index, and foci of microvascular proliferation; a diagnosis of L1CAM expressing, ZFTA:: NCOA1 fusion-positive supratentorial ependymoma (STE), and WHO grade 3 was rendered.
DISCUSSION
Ependymomas account for 4–8% of primary CNS neoplasms and are more common in children, where they account for 8–10% of CNS tumors. Shenoy et al. found an incidence of 2.5% in their study on ependymoma in India.[1] Ependymal neoplasms occur at all ages and include multiple tumor types. Before the 2016 update/fourth edition of the WHO classification of tumors of the CNS, ependymomas were classified as grades I, II, and III based on their grade of anaplasia. However, it was recognized that histological grading is insufficient in predicting outcome. Later, Pajtler et al.,[2] in their study, identified different molecular signatures in site-specific ependymomas and proposed nine distinct molecularly defined subgroups. These molecular subgroups show a close association with specific age groups. The 2021 WHO classification of tumors of the CNS, fifth edition (WHO CNS5), has introduced major changes including molecular profiles as the basis of diagnosis in CNS tumor classification. This edition focuses mainly on a layered reporting system/integrated diagnosis based on a holistic approach including histological findings, WHO grading, and IHC and molecular profiling. Ependymomas are now classified based on a combination of histopathologic features, anatomic site, and molecular profile, thus dividing them into different molecular subgroups across the supratentorial, posterior fossa, and spinal compartments. The molecular classification of ependymoma is based on novel diagnostic technologies such as deoxyribonucleic acid (DNA) methylome profiling and DNA sequencing.
The STEs are further subdivided into ZFTA fusion-positive (STE-ZFTA) and YAP1 fusion-positive ependymomas. ZFTA fusion tumors account for the majority of the STE and occur both in children and adults. The fusion of ZFTA gene with partner genes is believed to be the principal oncogenic driver of the disease. The most common gene partner is RELA; hence, previously these tumors were known as RELA-fusion-positive ependymomas.[4] However, recent studies have suggested that ZFTA-fusion may occur with other gene partners such as NCOA1/2, MAML2, and MN1.[5] In our case, a ZFTA:: NCOA1 fusion was detected.
STE-ZFTA fusion tumors are usually located in the frontal or parietal lobe and less commonly in the thalamus, or the region of hypothalamus or third ventricle. In our case, the mass involved the parieto-occipital lobe. Patients may present with focal neurological deficits, seizures, and raised intracranial pressure. On imaging, STEs generally appear as masses with irregular contrast enhancement. Intra-tumoral hemorrhage, cysts, and peritumoral edema are common.[4] Our patient’s MRI revealed an intra-axial mass with cystic and solid areas, bright post-contrast enhancement, and perilesional edema.
Macroscopic examination of STE-ZFTA-fusion tumors usually reveals a sharply demarcated tumor of soft consistency with commonly seen necrotic areas and dystrophic calcification. Histologic examination usually reveals tumor cells with uniform round nuclei, speckled chromatin, and poorly defined fibrillary cytoplasm. Psuedorosettes and true ependymal rosettes, although uncommon, may be present. The stroma shows a network of branching capillary blood vessels. Variant morphologies have also been described in the literature such as sarcoma-like, pleomorphic xanthoastrocytoma-like, high-grade glioma-like, malignant teratoma-like, embryonal tumor-like, and astroblastoma-like.[5,6] High-grade features constituting a WHO grade 3 tumor are brisk mitotic activity and microvascular proliferation. Cytologic anaplasia and necrosis are not sufficient to grade an ependymoma as WHO grade 3 tumor (anaplastic ependymoma is no longer considered a separate entity). Although histopathologic grading does not consistently relate to the overall outcome, grading is useful in guiding treatment when a molecular analysis is unavailable or unsuccessful. Our case revealed a largely circumscribed tumor with occasional ependymal canals and focal tanycytic differentiation. Brisk mitotic activity and microvascular proliferation amounting to a WHO grade 3 tumor were present.
On IHC, most STEs show immunoreactivity for S100 or GFAP with perivascular accentuation, perinuclear dot like expression of EMA, and focal OLIG-2 positivity. Studies by Tauziède-Espariat et al., Gessi et al., and others have found that p65 immunoexpression is highly correlated to the presence of RELA fusion, while most non- RELA STE-ZFTA tumors show L1CAM immunoexpression of varying degree and intensity. Thus, L1CAM IHC is a useful tool for recognizing STE in which ZFTA has non-RELA-fusion partners.[5-7] In our case, L1CAM was expressed. p65 IHC was not performed.
Surgery is the mainstay of treatment. Radiotherapy is recommended for grade 3 or incompletely resected grade II tumors. Chemotherapy is not useful as primary treatment and is commonly employed as salvage treatment for patients failing surgery and radiotherapy.[8] Complete surgical resection is the best predictor of long-term survival and a second look surgery for resection of residual tumor is increasingly advocated. Supratentorial location predicts poor outcome, as compared to posterior fossa or spinal location.[3,8]
STE with ZFTA fusion have the worst prognosis among all ependymomas. The prognosis of non-RELA ZFTA fusion ependymomas is even worse; however, STE with YAP fusions has a favorable outcome.[5,8] Our patient had a recurrence after just 2 months of surgery.
CONCLUSION
STE-ZFTA are uncommon neoplasms with poor prognoses. L1CAM IHC is a useful tool for recognizing STE in which ZFTA has non-RELA fusion partners. Timely histologic diagnosis and molecular analysis help in deciding the appropriate treatment in STE and predicting prognosis.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Ependymomas: A clinicopathologic study. Indian J Pathol Oncol. 2016;3:470-8.
- [CrossRef] [Google Scholar]
- Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27:728-43.
- [CrossRef] [PubMed] [Google Scholar]
- Central Nervous System Tumors In: WHO Classification of Tumors Series Vol 6. (5th ed). Lyon, France: International Agency for Research on Cancer; 2021. Available from: https://publications.iarc.fr/601
- [Google Scholar]
- MRI phenotype of RELA-fused pediatric supratentorial ependymoma. Clin Neuroradiol. 2019;29:595-604.
- [CrossRef] [PubMed] [Google Scholar]
- Supratentorial non-RELA, ZFTA-fused ependymomas: A comprehensive phenotype genotype correlation highlighting the number of zinc fingers in ZFTANCOA1/2 fusions. Acta Neuropathol Commun. 2021;9:135.
- [CrossRef] [PubMed] [Google Scholar]
- Ependymoma-like tumor with mesenchymal differentiation harboring C11orf95-NCOA1/2 or-RELA fusion: A hitherto unclassified tumor related to ependymoma. Brain Pathol Zurich Switz. 2021;31:e12943.
- [CrossRef] [PubMed] [Google Scholar]
- Role of immunohistochemistry in the identification of supratentorial C11ORF95-RELA fused ependymoma in routine neuropathology. Am J Surg Pathol. 2019;43:56-63.
- [CrossRef] [PubMed] [Google Scholar]
- Ependymoma: Evaluation and management updates. Curr Oncol Rep. 2022;24:985-93.
- [CrossRef] [PubMed] [Google Scholar]







