Translate this page into:
Immature teratoma of the ovary: A Pandora’s box-an oncologist’s perspective

*Corresponding author: Soumya Surath Panda, Department of Medical Oncology, Institute of Medical Sciences and Sum Hospital, Siksha “O” Anusandhan Deemed to be University, Bhubaneswar, Odisha, India. soumyasurathpanda@soa.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Sanyal A, Panda SS, Baisakh M, Panda AK, Mohanty SS, Naik S. Immature teratoma of the ovary: A Pandora’s Boxan oncologist’s perspective. Int J Mol Immuno Oncol. doi: 10.25259/IJMIO_1_2025
Abstract
Malignancy rarely arises in mature cystic teratoma; however, it tends to affect postmenopausal females – most commonly squamous cell carcinoma. Neural tissue may be found in some cases, although very rarely seen in immature teratoma. We present a case report of a 22-year-old nulliparous female with left ovarian immature teratoma – Stage-IA, Grade-3 with oligodendroglial differentiation. The case was discussed in the multidisciplinary tumor board - the patient received four cycles of systemic therapy and is now disease-free at 9 months follow-up.
Keywords
Adolescent and young adults
Germ cell tumors
Immature teratoma
Oligodendroglioma
Ovarian neoplasms
INTRODUCTION
Affecting the postmenopausal population, a mature cystic teratoma typically harbors squamous cell carcinoma as the most common component.[1] However, other elements of differentiation have been previously reported.[2] Evidence-based management is the motto of oncology, even for the rarest malignancies. Therefore, it is prudent to discuss the dilemma of immature teratoma that affects the adolescent and young adult age group – a nation’s future and, in our case, our patient.
In this case report, we focused on bringing a more comprehensive and practical approach to managing a case of immature teratoma with oligodendroglial differentiation in a young lady diagnosed in the 2nd decade of her life. The Institutional Review Board approved this study, and the patient’s consent was obtained.
CASE REPORT
A 22-year-old unmarried, nulliparous female presented to our center in post-operative status with the diagnosis of an immature teratoma. The patient had initially presented with abdominal distension and pain in the lower abdomen ×3 months. Baseline tumor markers were normal. Contrast-enhanced computed tomography of the abdomen had depicted a large cystic mass in the abdominopelvic region ~17 cm, arising from the left ovary. She had undergone exploratory laparotomy and left salpingo-oophorectomy in May 2023. The post-operative histopathology report (HPR) suggested a Grade-3 left ovarian immature teratoma of size ~15 cm, with oligodendroglial differentiation; ovarian surface and fallopian tube were free of deposits – pT1a (IA), [Figure 1a-e]. After a thorough discussion in the multidisciplinary tumor board (MDT) – National Comprehensive Cancer Network guidelines were discussed after a brief review of the literature; given Grade-3 disease, the board decided to proceed with three cycles of BEP (Inj. Bleomycin 30 IU on days – 1,8,15, Inj. Etoposide @ 100 mg/m2/day × 5 days, Inj. Cisplatin @20 mg/m2 day × 5 days) every 21 days, followed by one cycle of EP as per protocol. Post-operative imaging suggested no residual/recurrent disease with normal tumor markers. Before treatment initiation, the option of fertility preservation was discussed; however, the patient and her respondents were unwilling and signed a written consent. The patient completed three cycles of BEP. Post-3rd cycle, she was admitted for febrile neutropenia with Grade-2 diarrhea and was managed symptomatically with i/v antibiotics, antidiarrheal measures, and supportive care. She completed cycle 4 with EP (25% dose reduction) on September 27, 2023. Tumor markers were normal post-chemotherapy completion. The case was re-discussed in MDT, and the patient was planned to be kept on 3-monthly follow-up for the first 2 years – monitoring with ultrasonography whole abdomen and pelvis and serum tumor markers and then every 4th–6th monthly till the 5th year.
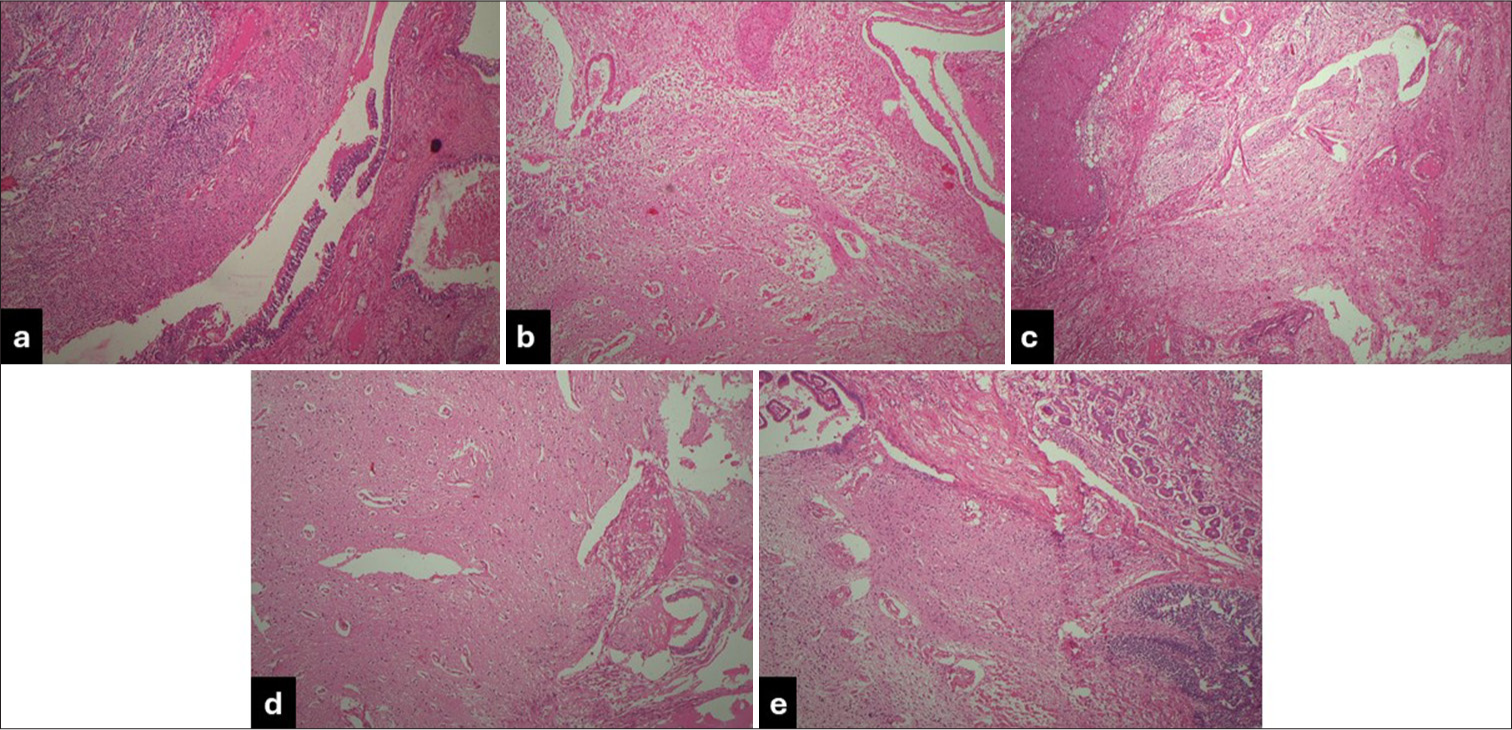
- Left salpingo-oophorectomy specimen: (a) Focal areas of moderately cellular neoplasm composed of round to oval tumor cells with mild pleomorphic vesicular nuclei; (b) scanty to moderate cytoplasm and perinuclear halo arranged in sheets and singles against a glio-fibrillary background; and (c) intervening stroma with frequent thin-walled branching blood vessels; (d and e). No significant mitosis, IMCT Grade-3. (200X; Hematoxylin and eosin stain).
The patient has a disease-free survival of 9 months at the time of writing this paper. The last follow-up was in May 2024, and the previous telephonic update was in June 2024.
DISCUSSION
Squamous cell carcinoma is the most common malignancy arising in a mature cystic teratoma; it tends to affect the postmenopausal population.[1] Other elements of differentiation, including but not limited to gastrointestinal adenocarcinoma, sarcoma, and melanoma, have also been reported as part of various case reports.[2]
The presentation of our patient at the age of 22 years with abdominal pain corresponds to the previously reported young adult age group and chief complaint;[1-5] however, its diagnosis in the adolescent age group has also been documented.[6]
As part of primary treatment, our patient underwent exploratory laparotomy with the left salpingo-oophorectomy. On HPR, it was found to be a cystic ovary ~15 cm tumor size in largest dimension, Grade-3, with the typical histopathological finding of round to oval tumor cells having mild pleomorphic vesicular nuclei, scanty to moderate cytoplasm and perinuclear halo (fried egg appearance), arranged in sheets and singles against a glial fibrillary background, and frequent thin-walled branching blood vessels. All the microscopic findings correspond to previously published reports.[1-6] The patient tolerated three cycles of BEP and one cycle of EP with a manageable toxicity profile and was disease-free for 9 months; however, previously reported cases received systemic therapy at the time of relapse and succumbed to the disease eventually.[4] A table depicting the relevant findings from the present case report with other reported cases and a case series has been summarized below for the readers [Table 1].
| Author (Year of publication and country of origin) | Reference No. (except our study) | Type of study and relevant findings (DFS at the time of last follow-up) |
|---|---|---|
| Sanyal et al. (Our Study) (India) | - | Case Report; 22-year-old nulliparous female Underwent left salpingo-oophorectomy→HPR s/o Stage-IA Grade-3 immature teratoma with oligodendroglial component Received: BEP×3 cycles→EP×1 cycle DFS→9 months |
| Zannoni et al. (2002) (Italy) | [1] | Case Report; 29-year-old nulliparous female Underwent Laparotomy with the right ovariectomy HPR s/o mature cystic teratoma with oligodendroglial component IHC: GFAP, NFP+/Leu-7 focally+/NSE, Synaptophysin – DFS→48 months |
| Caltabiano and Lanzafame (2008) (Italy) | [2] | Case Report; 18-year-old female HPR s/o immature teratoma with oligodendroglial component IHC: S100+/GFAP, NSE, Synaptophysin – DFS→24 months |
| Opris et al. (2009) (France) | [3] | Case Report; 29-year-old multiparous female Underwent laparotomy with left ovariectomy HPR s/o mature cystic teratoma with oligodendroglial component IHC: GFAP, NFP+/Synaptophysin–/Ki-67: 3% RT-PCR: LOH on Chromosomes 10q and 19q13 DFS→10 months |
| Bay et al. (2010) (Turkey) | [6] | Case Report; 13-year-old female Underwent oophorectomy HPR s/o mature cystic teratoma with oligodendroglial component IHC: S100+/GFAP, Synaptophysin–/Ki-67: 2% DFS→9 months |
| Ud Din et al. (2012) (Pakistan) | [4] | Retrospective case series of 6 cases; Age group: 12–28 years (Mean 21 years) All underwent surgery HPR s/o 2 mature and 4 immature cystic teratoma with oligodendroglial component; all WHO Grade-2 IHC: Synaptophysin–/Ki-67: 2–4% DFS range→1–42 months (2 Immature teratoma cases: OS→1 and 36 months) |
| Ünal et al. (2014) (Turkey) | [5] | Case Report; 18-year-old female Underwent laparotomy+right oophorectomy HPR s/o mature cystic teratoma with oligodendroglial component IHC: GFAP, EGFR, p53+/Ki-67: 2–5% DFS→13 months |
HPR: Histopathology report, DFS: Disease-free survival, GFAP: Glial fibrillary acidic protein, IHC: Immunohistochemistry, EGFR: Epidermal growth factor receptor, NFP: Neurofilament protein, NSE: Neuron-specific enolase, WHO: World Health Organization, BEP: Bleomycin, etoposide and platinum, BEP: Bleomycin, etoposide and platinum, RT-PCR: Reverse transcription-Polymerase chain reaction, LOH: Loss of heterozygosity.
Despite our report being limited to a single patient’s data, we cannot undermine that it helped us to understand the dictum “the eyes don’t see what the mind doesn’t know” – this rarest form of presentation of immature teratoma with oligodendroglial differentiation. An early surgical intervention followed by systemic therapy with BEP, in this case of Stage-IA Grade-3, is prudent to prevent relapse and or recurrence for better survival outcomes. This entity is sensitive because this is the age group of women that constitutes the fertile population and thereby affects all aspects of health. Ultimately, the goal is to help achieve an overall normal life expectancy, a good quality of life, and fertility preservation through oocyte banking and early surgical intervention, as most cases are localized to a single ovary.
New knowledge and learning points
Oligodendroglial differentiation in immature teratoma is a rare finding, indicating poor overall survival.
The oligodendroglial component will not change our treatment approach.
As per established guidelines, these patients will inadvertently benefit from adjuvant BEP chemotherapy.
CONCLUSION
Our case highlights the importance of rigorous histological and immunohistochemical examination in patients with immature teratoma. As rare as it may be, such specimens can harbor neural tissue elements, including the rarest of them all, an oligodendroglioma. These patients should be promptly treated based on the stage and grade of the mature/immature teratomas as per institutional guidelines since neural elements, especially oligodendroglial components, are a harbinger of worse prognosis and should be nipped at its bud to improve the survival outcomes.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil
References
- Oligodendroglioma arising within a mature cystic ovarian teratoma: Case report and review of the literature. Acta Obstet Gynecol Scand. 2002;81:896-7.
- [CrossRef] [PubMed] [Google Scholar]
- Oligodendroglioma arising in an immature ovarian teratoma: Case report. Pathologica. 2008;100:420-3.
- [Google Scholar]
- Oligodendroglioma arising in an ovarian mature cystic teratoma. Int J Gynecol Pathol. 2009;28:367-71.
- [CrossRef] [PubMed] [Google Scholar]
- Oligodendroglioma arising in the glial component of ovarian teratomas: A series of six cases and review of literature. J Clin Pathol. 2012;65:631-4.
- [CrossRef] [PubMed] [Google Scholar]
- Oligodendroglioma arising in mature cystic teratoma. Case Rep Oncol Med. 2014;2014:745462.
- [CrossRef] [PubMed] [Google Scholar]
- Oligodendroglioma arising in a mature cystic ovarian teratoma in a child. Pediatr Hematol Oncol. 2010;27:636-40.
- [CrossRef] [PubMed] [Google Scholar]







