Translate this page into:
Ibrutinib and atrial fibrillation as cardiotoxicity – A new safety warning that impacts overall survival

*Corresponding author: Purvish Parikh, Mumbai Oncocare Centers, Mumbai, Mumbai, Maharashtra, India. purvish1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Parikh P, Aggarwal IB, Daddi A, Narasimhan PN, DSouza HH, Agarwal V, et al. Ibrutinib and atrial fibrillation as cardiotoxicity – A new safety warning that impacts overall survival. Int J Mol Immuno Oncol 2022;2:54-7.
Abstract
Atrial fibrillation is a well recognized side effects of several drugs. However it is ignored since most studies have failed to show that it impacts survival adversely. This is not the case with ibrutinib, especially amongst patients with pre-existing cardiac morbidities. In this article, we provide practical consensus guidelines for cancer patients being commenced on therapy with ibrutinib.
Keywords
Burton’s tyrosine kinase inhibitors
Hematological malignancies
Bleeding
Hypertension
Adverse drug reaction
Targeted therapy
BURTON’S TYROSINE KINASE (BTK) INHIBITORS AND B CELL HAEMATOLOGICAL MALIGNANCIES
Inhibition of BTK is effective at inhibiting B-cell neoplasm growth.[1] Ibrutinib became the first BTK inhibitor improving progression-free as well as overall survival in de novo and relapsed/refractory chronic lymphocytic leukemia (CLL). Similar efficacy was also shown in other B cell malignancies (mantle cell lymphoma; Waldenström’s macroglobulinemia; and marginal zone lymphoma).[1-3]
IBRUTINIB, THE FIRST BTK INHIBITOR, CEMENTS ITS ROLE IN THE MANAGEMENT OF B CELL MALIGNANCIES WITH BETTER OVERALL SURVIVAL
RESONATE, a phase 3 study amongst patients with CLL/SLL (which included patients with high risk) demonstrated that ibrutinib monotherapy was better than ofatumumab.[4]
At a median follow-up of 65.3 months, the overall response rate (ORR) was 91% with ibrutinib. Median progression-free survival (PFS) was 44.1 months with ibrutinib as compared to 8.1 months with ofatumumab (P < 0.001; hazard ratio: 0.148; 95% confidence interval [CI]: 0.113–0.196). The benefit was also seen amongst the high-risk patients [del(17p), TP53 mutation, del(11q), and/or unmutated IGHV status]. This translated into better overall survival (censored for a crossover) with ibrutinib versus ofatumumab. This study also reported that there were no new safety signals and that adverse event-related discontinuation of ibrutinib was seen in only 16% of patients. No wonder this became a landmark study for use of ibrutinib in B cell malignancies [Figure 1].
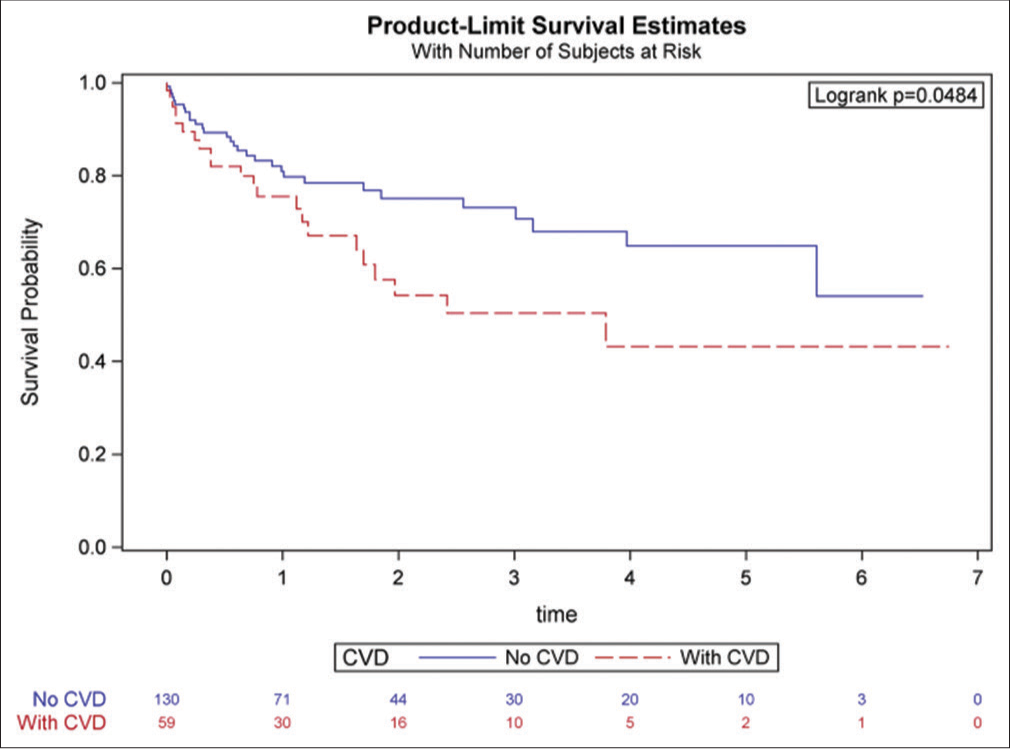
- Survival probability among cancer patients treated with ibrutinib – with and without pre-existing cardiovascular disease (Reproduced from Avalon JC, Fuqua J, Miller T, Deskins S, Wakefield C, King A, Inderbitzin-Brooks S, Bianco C, Veltri L, Fang W, Craig M, Kanate A, Ross K, Malla M, Patel B. Pre-existing cardiovascular disease increases the risk of atrial arrhythmia and mortality in cancer patients treated with Ibrutinib. Cardiooncology 2021 Nov 19;7(1):38. doi: 10.1186/s40959-021-00125-8. PMID: 34798905; PMCID: PMC8603583.).
RED FLAG REGARDING CARDIOVASCULAR TOXICITY WITH BTK INHIBITORS
Some studies started reporting cardiac adverse reactions – the initial focus was on the crucial five - atrial fibrillations, bleeding, cardiac failure, hypertension, and ventricular tachycardia. As more and more data got published, it became clear that atrial fibrillation, bleeding, and hypertension was more common than cardiac failure or ventricular tachycardia. There was initial complacency because; historically TKIs have increased atrial fibrillation without significant serious consequences. Maturing data seemed to confirm the same – while atrial fibrillation was found to be higher, there seemed to be no adverse impact on overall survival.[5-7] Part of the complacency was also because the only approved BTK inhibitor (i.e., Ibrutinib) gave results far superior to other options, especially in CLL.
REVISITING IBRUTINIB SAFETY
When second-generation inhibitors (showing more selective BTK inhibition) became available, the scenario changed dramatically. We began to realize that acalabrutinib and zanubrutinib[8-11] were associated with lower cardiovascular side effects. This made it vital to revisit the safety profiles of BTK inhibitors and compare their differences.[12]
In retrospect, almost none of the BTK inhibitor studies required systematic screening for AF. As we know, AF occurs paroxysmal – the incidence captured directly being proportional to the intensity of screening. The first inkling of the real occurrence was reported in a prospective study involving 53 patients with a B cell malignancy who received ibrutinib. These patients (whose screening involved documentation with palpation of the pulse and electrocardiography every 3 months) revealed the true incidence of ibrutinib-related AF of 23% (95% CI 9–35%) at 12 months – almost one in four patients![13]
WHY ARE FIRST AND SECOND-GENERATION BTK INHIBITORS HAVING DIFFERENT CARDIOTOXICITY?
The short answer is we are not sure. Cardiotoxicity, especially AF, can occur through the BTK-mediated pathway or off-target pathways (the Tec non-receptor tyrosine kinase pathway) - because both are expressed in cardiac tissue. The BTKs regulate the phosphoinositide 3-kinase (PI3K)-Akt pathway[14] whereas the Tec pathway is likely to be involved in cardiac response to stress.[15] Interestingly, patients who develop AF have significantly lower cardiac PI3K-Akt activity.[16] Ibrutinib targets the alpha-subunit of P13K (the predominant P13K isoform expressed in cardiovascular tissues) and could predispose toward atrial fibrosis, which is a hallmark of AF. The second-generation BTK inhibitors (acalabrutinib and zanubrutinib) have a more specific action on the BTK pathway and hence have reduced side effects, especially cardiotoxicity.
WHAT’S THE REAL DEAL ABOUT IBRUTINIB INDUCED AF?
It was the general impression that AF as a known side effect was more of paper toxicity. This was for two reasons. The first was that the occurrence of AF was not associated with any impact on the outcome, especially overall survival.[17,18] The second was that there were conflicting reports on whether the treatment of AF was beneficial or harmful.[19,20]
The final analysis of RESONATE study by Munir et al. opened our eyes, changed our understanding, and is sufficiently profound to be considered a landmark manuscript.[4]
To recapitulate, the RESONATE study was the first open-labeled, multicenter, randomized phase 3 study (comparing ibrutinib to ofatumumab) in previously treated patients with CLL/SLL that showed benefit in all three key outcome parameters – OS, PFS, and ORR. No wonder Ibrutinib received full FDA following availability of this data. The first report analysis also confirmed that it was generally well-tolerated.
Patients were randomized in a 1:1 fashion. Those in the study arm received oral ibrutinib 420 mg once daily (until disease progression or unacceptable toxicity). With an Ibrutinib arm median follow-up of 65.3 months (range: 0.3−71.6); median duration of ibrutinib therapy of 41 months (range: 0.2–71.1); and ibrutinib being taken for more than 4 years by 41% of patients, this is solid data and analysis. Over the 71 months treatment duration, commonly reported grade ≥3 AEs included neutropenia (25%), pneumonia (21%), thrombocytopenia (10%), anemia (9%), hypertension (9%), urinary tract infection (7%), diarrhea (7%), and atrial fibrillation (6%).
For the rest of this manuscript, we will limit our discussion to the impact of Ibrutinib on AF only. Recently, Patel et al. have elegantly reported on the impact of AF on mortality in West Virginia University cancer patients treated with Ibrutinib.[21]
They analyzed their single-institution data collected between 2012 and 2020. Patients were divided into two groups - those with pre-existing CVD (known history of coronary artery disease, heart failure, pulmonary hypertension, moderate valvular heart disease, and cardiovascular device implantation) and those without CVD. The primary outcome evaluated was an incidence of atrial arrhythmia, and the secondary outcomes included all-cause mortality. A total of 217 patients were included in the study. The incidence of new-onset atrial arrhythmia was 17% in the pre-existing CVD arm as compared to 7% in the without CVD arm (P = 0.02). Patients with pre-existing CVD also had higher all-cause mortality (OR 1.9, 95% CI 1.06–3.41, P = 0.01) and reduced chance of survival (43% vs. 54%, P = 0.04). Interestingly, the risk of bleeding was similar in both arms.
RECOMMENDED PRACTICAL CONSENSUS GUIDELINES FOR CANCER PATIENTS BEING COMMENCED ON IBRUTINIB
All cancer patients being considered for ibrutinib therapy should be evaluated for pre-existing cardiovascular conditions or risk factors
Data from prospective randomized clinical trials have enabled us to arrive at guidelines for the treatment of AF. Unfortunately, most of these studies have deliberately excluded cancer patients. If cancer patients develop AF for the 1st time, their risk of thromboembolism is double. Since cancer itself could simulate a prothrombotic state, the conventional scoring system cannot reliably predict how to manage them appropriately
Thyroid dysfunction could also add to the risk of AF
-
If pre-existing cardiovascular disease exists
Their treatment should be optimized as required
Their monitoring while on Ibrutinib therapy should be increased (every 3 months or as appropriate) – especially during the 1st year of therapy.
Physical pulse
Electrocardiography
Holter monitoring might be required in special circumstances
As AF occurs paroxysmal, without systematic screening, the occurrence might be missed
Ibrutinib may need to be dose reduced or discontinued in case of red flags
Amongst patients without pre-existing cardiovascular disease, the risk of ibrutinib associated AF is less, but is still higher than in cancer patients not on ibrutinib
If the patients require initiation of anticoagulation, the risk, and benefits of thrombosis versus bleeding need to be considered. Use of low molecular weight heparin, when required, is considered safe
-
If AF needs medical treatment, keep in mind potential Ibrutinib drug interactions
Ibrutinib is metabolized by cytochrome P450 - CYP3A and CYP2D6. If drugs affect CYP3A activity (increase or decrease) metabolism of ibrutinib will change, efficacy may reduce or toxicity may increase. In addition, ibrutinib can increase the potential risk of bleeding (on its own without the patient simultaneously receiving any anticoagulants) because of its action on platelet-signaling pathways. A small percentage of patients receiving ibrutinib have also been documented to have thrombocytopenia
Amiodarone, used to treat arrhythmia, can inhibit CYP3A4, which, in turn, would increase circulating ibrutinib levels and thereby enhance the toxicity
Ibrutinib alters CYP3A4, which potentially can lead to higher circulating levels of warfarin
Ibrutinib has been known to elevate plasma levels of dabigatran (a direct thrombin inhibitor) because it is a PGP (P-glycoprotein) substrate (independent of CYP450 interaction). This can also increase the risk of bleeding
Similarly, through PGP inhibition, ibrutinib can lead to elevated levels of digoxin, increasing its toxicity
Whether ibrutinib has any effect on factor Xa inhibitors such as edoxaban, apixaban, and rivaroxaban is still uncertain
Beta-blockers show an absence of any drug-drug interaction with ibrutinib and are the preferred first-line agents for control of ventricular rate control in patients with AF
While diltiazem and verapamil (calcium-channel blockers) are also effective in AF, they inhibit CYP450 3A4. So their use in patients receiving ibrutinib might increase circulating plasma levels of ibrutinib, with associated toxicity.
Patients with multiple risk factors for AF (such as old age [>60 years], hypertension, obstructive sleep apnea, coronary artery disease, cardiomyopathy, heart failure, thyroid or lung disease) could benefit from a multidisciplinary/cardiooncology tumor board or consultation (where available and when appropriate), before the start of ibrutinib therapy.
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: An international, randomized, open-label, phase 3 study. Lancet. 2016;387:770-8.
- [CrossRef] [Google Scholar]
- Ibrutinib monotherapy in symptomatic, treatment-naïve patients with Waldenström macroglobulinemia. J Clin Oncol. 2018;36:2755-61.
- [CrossRef] [PubMed] [Google Scholar]
- Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517-28.
- [CrossRef] [PubMed] [Google Scholar]
- Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94:1353-63.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of atrial fibrillation in ibrutinib-treated CLL patients: A prospective study. J Hematol Oncol. 2018;11:79.
- [CrossRef] [PubMed] [Google Scholar]
- Management of atrial fibrillation in patients on ibrutinib: A Cleveland clinic experience. Cureus. 2018;10:e2701.
- [CrossRef] [Google Scholar]
- Ibrutinib-related atrial fibrillation: A single-center Australian experience. Asia Pac J Clin Oncol. 2019;15:e187-90.
- [CrossRef] [PubMed] [Google Scholar]
- ASCEND Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38:2849-61.
- [CrossRef] [PubMed] [Google Scholar]
- Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukemia (ELEVATE TN): A randomized, controlled, phase 3 trial. Lancet. 2020;395:1278-91.
- [CrossRef] [Google Scholar]
- Acalabrutinib (ACP-196): A covalent Bruton's tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363:240-52.
- [CrossRef] [PubMed] [Google Scholar]
- Bruton's tyrosine kinase inhibitors and cardiotoxicity: More than just atrial fibrillation. Curr Oncol Rep. 2021;23:113.
- [CrossRef] [PubMed] [Google Scholar]
- High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart. 2019;6:e001049.
- [CrossRef] [PubMed] [Google Scholar]
- Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829-30.
- [CrossRef] [PubMed] [Google Scholar]
- Discovery of (R)-1-(3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d] pyrimidin-1-yl)piperidin-1-yl)-2-(dimethylamino)ethanone (CHMFL-FLT3-122) as a potent and orally available FLT3 kinase inhibitor for FLT3-ITD positive acute myeloid leukemia. J Med Chem. 2015;58:9625-38.
- [CrossRef] [PubMed] [Google Scholar]
- Phosphoinositide 3-kinase p110? is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ Heart Fail. 2012;5:523-34.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-induced atrial fibrillation. J Am Coll Cardiol. 2004;44:2117-24.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-induced arrhythmias: A scientific statement from the American heart association. Circulation. 2020;142:e214-33.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of AFib: Is Ablation That Good or is Drug Therapy That Bad? Available from: https://www.acc.org/latest-in-cardiology/articles/2014/07/18/15/17/treatment-of-afib-is-ablation-that-good-or-is-drug-therapy-that-bad [Last accessed on 2022 Jan 04]
- [Google Scholar]
- Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA. 2019;321:1261-74.
- [CrossRef] [PubMed] [Google Scholar]
- Pre-existing cardiovascular disease increases the risk of atrial arrhythmia and mortality in cancer patients treated with Ibrutinib. Cardiooncology. 2021;7:38.
- [CrossRef] [PubMed] [Google Scholar]






