Translate this page into:
Gastric cancer or hodgkin lymphoma? Unraveling the diagnostic dilemma of primary gastric classical hodgkin lymphoma

*Corresponding author: Soumya Surath Panda, Department of Medical Oncology, Institute of Medical Sciences and Sum Hospital, Siksha ‘O’ Anusandhan Deemed to be University, Bhubaneswar, Odisha, India. soumyasurathpanda@soa.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Meedimale S, Moharana L, Mohanty SS, Samantaray L, Panda SS. Gastric cancer or hodgkin lymphoma? Unraveling the diagnostic dilemma of primary gastric classical hodgkin lymphoma. Int J Mol Immuno Oncol. 2025;10:41-4. doi: 10.25259/IJMIO_28_2024
Abstract
Primary gastric classical Hodgkin lymphoma (PGHL) is a primary malignant neoplasm of the stomach, often clinically and radiologically mimicking gastric cancer. Immunohistochemistry is needed to diagnose such rare tumors. A 45-year-old male presented with abdominal pain, nausea, and unintentional weight loss. First investigations, such as endoscopic examination and biopsy, indicated that this was a case of non-Hodgkin lymphoma (HL). However, the immunohistochemistry (IHC) reinforced the classical HL with positive markers of CD15, CD30, PAX5, and MUM1, categorizing it as a mixed cellularity subtype. The patient underwent distal gastrectomy with Billroth II reconstruction and received four cycles of bleomycin, doxorubicin, vinblastine, dacarbazine chemotherapy, leading to complete clinical and radiological remission. PGHL, although rare, should be considered in the differential diagnosis of gastrointestinal malignancies. Immunohistochemistry plays a pivotal role in correct diagnosis, and a combination of surgery and chemotherapy is the ideal treatment strategy, as demonstrated in this case.
Keywords
Gastric cancer
Immunohistochemistry
Mixed cellularity subtype
Primary gastric Hodgkin lymphoma
INTRODUCTION
Lymphoma, which usually originates from the lymphatic system, can also involve the gastrointestinal (GI) tract when the disease is usually disseminated. Around 40% of the extra-nodal involvement of the lymphomas are from the GI tract[1] with Non-hodgkin type (NHL), where the stomach is involved in the majority of patients, accounting for 60% of cases, followed by the small intestine and large intestine in 30% and 20% cases, respectively.[2,3]
In contrast to NHL, hodgkin lymphoma (HL) usually involves cervical lymph nodes. The primary gastric classical hodgkin lymphomas (PGHL) without nodal involvement are rare.[4] Because of the low suspicion of this type of cancer, along with the similarity in the clinical and radiological picture of gastric cancers, IHC plays a pivotal role in the confirmation of these cases. Only a few case series and reports have been published in the literature. Here, we describe a case of 45-year-old male patient with no palpable peripheral lymph nodes and mimicking gastric cancer who was surprisingly diagnosed as classical PGHL. The institutional review board approved this study and the patient’s consent was taken.
CASE REPORT
A 45-year-old man reported experiencing abdominal pain, nausea, and a significant weight loss of approximately 4–5 kg over the past 3 months. At presentation, he had no history of vomiting, hematemesis, or melena. He has no comorbidities such as diabetes mellitus, hypertension, or other significant family history of hereditary cancers.
The patient first visited a local hospital for acute abdominal pain and nausea and was on symptomatic treatment for a month. As his symptoms did not subside, he visited our hospital, one of the tertiary care centers, where he was advised for an ultrasound of the abdomen, which revealed circumferential asymmetrical wall thickening in the anthropologic region of the stomach measuring up to 14 mm with perigastric lymph nodes along the lesser and greater curvature. This again was confirmed by contrast-enhanced computed tomography of the abdomen.
He was then planned for upper GI endoscopy, where an ulceroproliferative growth was observed in the distal body, involving the lesser curvature and antrum, with the final diagnosis indicating carcinoma of the stomach. The biopsy from the growth revealed NHL and was advised for extended panel IHC. During this time, the patient underwent whole-body positron emission tomography-computed tomography because of lymphoma suspect, where mildly fluorodeoxyglucose (FDG)-avid irregular wall thickening is seen involving the body and antrum of the stomach with wall thickening of around 1.4 cm and standard uptake value max of 5.1 [Figure 1]. There were also a few inflammatory mildly FDG-avid inguinal lymph nodes.
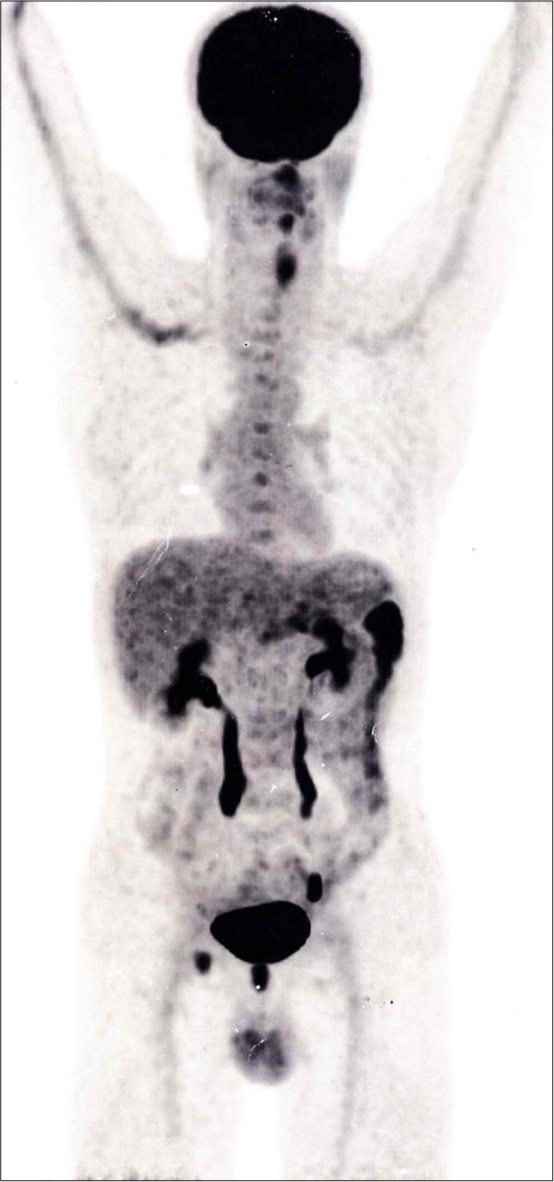
- Positron emission tomography-computed tomography ➜ FDG-avid irregular wall thickening involving the body and antrum of the stomach. FDG: Fluorodeoxyglucose.
IHC report [Figure 2] surprisingly was compatible with HL, classical type mixed cellularity variant. The markers such as CD15, CD30, CD20, CD3, CD4, CD8, BCL2, and MUM1 were strongly positive and markers such as BCL6, EMA, CD5, CD23, CYCLIN D1, and ALK1 were negative. The leukocyte common antigen was negative in large cells but strongly diffusely positive in background B and T cells. The case was discussed in a multidisciplinary tumor board, following which the patient, diagnosed with PGHL (stage IE), was referred to a gastrosurgeon, where he was evaluated, and distal gastrectomy, Bill Roth II reconstruction, was performed. The histopathological specimen obtained from surgery confirmed lymphoma of the hodgkin variety arising from the stomach. Following surgery, he recovered quickly and was planned for standard Bleomycin-DacarbazineDoxorubicin-Vinblastine protocol. Post four rounds of chemotherapy, the patient had complete radiological as well as clinical response. He was on regular follow-up for 3-year post-completion of chemotherapy but was lost to follow-up thereafter.
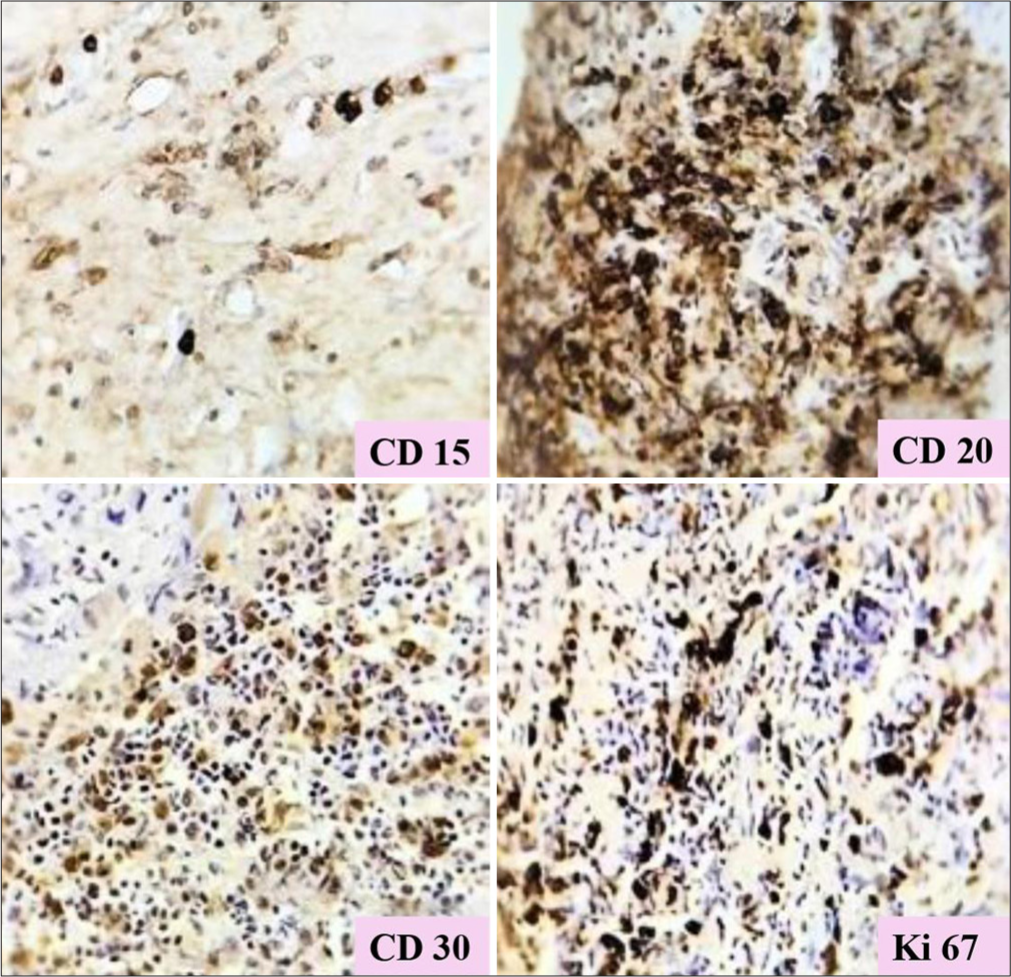
- Immunohistochemistry showing strong positivity of CD15, CD20, CD30, Ki67. 20X
DISCUSSION
Lymphoma, usually a lymphatic system disease, can also be seen in the GI tract. Most lymphomas from the digestive system are of non-hodgkin variety,[5] with the stomach being the most common involved organ and the esophagus the rarest.[6]
Around 40% of extranodal lymphomas seen primarily in the GI tract are of NHL type and comprise 4% of all GI malignancies.[7] The primary GI-HL is a rare entity. It comprises <1% of all the lymphomas in the GI tract.[4,8] Only a few cases are documented in the literature, highlighting its rare incidence [Table 1].
| Researcher | References | Time frame | Positive markers | Negative markers |
|---|---|---|---|---|
| Saito et al. | [11] | 2007 | CD30, Oct2, Bob1 | EMA, CD15 |
| Jung et al. | [4] | 2012 | CD79, CD15, CD30 | EMA, ALK, c-KIT |
| Sethi et al. | [12] | 2015 | CD15, CD30, PAX5 | CD20, CD45 |
| Babu et al. | [13] | 2019 | CD30, MUM1, PAX5 | CD3, CD20, CD15, LMP1 |
| Alshawkani et al. | [14] | 2021 | CD30, CD15, CD45, CD79a, PAX5 | CK7, CAM5.2 |
| Krishnan et al. | [15] | 2022 | CD30, CD15 | CD20, CD45, CD3, CK, ALK |
| Batiuk et al. | [16] | 2023 | CD15, CD30, CD79a, PAX5 | CD19, CD22, CD43, ALK |
| Kuan et al. | [17] | 2023 | CD30, PAX5, MUM1 | CD3, CD5, CD7 |
| Current case | - | - | CD15, CD30, CD20, LCA, PAX5, BCL2, BCL6, MUM1, C-MYC | CD5, CD23, Cyclin D1, ALK1 |
PGHL: Primary gastric classical hodgkin lymphoma, IHC: Immunohistochemistry
Previous studies reported HL in 2–9% of cases; however, upon review with IHC, these cases were later reclassified as NHL.[9] In our case, the patient initially presented with features suggestive of gastric cancer but was diagnosed as HL based on histopathology and IHC findings, thereby establishing the importance of IHC in diagnosing such peculiar cases without any dilemma.
Primary extranodal lymphomas are common in the stomach. Dawson et al.[10] introduced diagnostic criteria for HL originating from the stomach in 1961, which even now is valid with little modifications and is used routinely in clinical practice. The following Table 2 summarizes the Dawson criteria for HL originating from the stomach:
| Criterion | Description |
|---|---|
| Primary tumor location | The tumor’s primary location is in the GI tract, regardless of the involvement of adjacent lymph nodes. |
| Mediastinal lymph nodes | No mediastinal lymph node involvement |
| Peripheral lymph nodes | Peripheral lymph nodes remain uninvolved at the time of diagnosis. |
| Liver, spleen, bone marrow | Liver, spleen, and bone marrow show no signs of involvement |
| Routine blood results | Routine blood test results are within normal limits |
PGHL: Primary gastric classical hodgkin lymphoma, GI: Gastrointestinal
It is difficult to diagnose gastric HL based on histopathology, as in our case, after doing the upper GI endoscopy, which was suggestive of carcinoma stomach based on ulcer proliferative growth at an antropyloric location. The histopathology report was suggestive of NHL and, certainly, after immunohistochemistry, confirmed to be lymphoma of the stomach of the hodgkin variety. Based on CD 30, the diagnosis of HL cannot be established as it can also be expressed in lymphomas such as peripheral T cell variant, anaplastic, and primary cutaneous lymph proliferative disorders. Hence, the extended panel of IHC is always needed to confirm the HL of the stomach and was done in our case to confirm the diagnosis.
PGHL treatment is not standardized because of the rare incidence. The literature suggests that the ideal approach of primary surgery followed by adjuvant chemotherapy to address systemic disease is the best treatment to date.[18] Some case reports even suggest preoperative chemotherapy. In our case, the patient underwent distal gastrectomy, received four cycles of post-operative ABVD chemotherapy, and has been on follow-up for three years.
CONCLUSION
The HL of the stomach, clinical and radiological picture mimics the stomach cancer. Although the extranodal involvement of HL is rare, it should be one of the differentials so that it is not misdiagnosed. The role of extended panel IHC comes into the picture to confirm such rare cases. As per the available literature, primary surgery followed by adjuvant ABVD protocol is the best possible treatment to which patients respond exceptionally well, as was in our case.
Acknowledgment
The authors acknowledge the Department of Pathology, Institute of Medical Sciences and Sum Hospital.
Author contributions
All authors contributed equally and did the critical revision of the final manuscript. All authors read and approved the final manuscript.
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Dysphagia revealing esophageal involvement by non-Hodgkin's lymphoma. Ann Hematol. 2005;84:482-3.
- [CrossRef] [PubMed] [Google Scholar]
- Primary gastrointestinal non-Hodgkin's lymphoma of the small and large intestines: A systematic review. J Gastrointest Surg. 2016;20:827-39.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal lymphoma: Recent advances in diagnosis and treatment. Digestion. 2013;87:182-8.
- [CrossRef] [PubMed] [Google Scholar]
- Primary gastric Hodgkin's lymphoma. J Korean Surg Soc. 2012;83:111-4.
- [CrossRef] [PubMed] [Google Scholar]
- Gastric MALT lymphoma-update on diagnosis and treatment. Best Pract Res Clin Gastroenterol. 2014;28:1069-77.
- [CrossRef] [PubMed] [Google Scholar]
- Primary gastric lymphoma pathogenesis and treatment: What has changed over the past 10 years? Br J Haematol. 2007;136:521-38.
- [CrossRef] [PubMed] [Google Scholar]
- Primary extranodal lymphomas of stomach: Clinical presentation, diagnostic pitfalls and management. Ann Oncol. 2008;19:1992-9.
- [CrossRef] [PubMed] [Google Scholar]
- Primary Hodgkin's disease of the stomach. Am J Clin Pathol. 1988;89:806-9.
- [CrossRef] [PubMed] [Google Scholar]
- Primary gastrointestinal lymphoma. A clinicopathologic study of fifty-seven cases. Cancer. 1983;51:701-11.
- [Google Scholar]
- Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80-9.
- [CrossRef] [PubMed] [Google Scholar]
- Primary gastric Hodgkin's lymphoma expressing a B-Cell profile including Oct-2 and Bob-1 proteins. Int J Hematol. 2007;85:421-5.
- [CrossRef] [PubMed] [Google Scholar]
- Primary gastric Hodgkin's lymphoma: An extremely rare entity and a diagnostic challenge. Dig Dis Sci. 2015;60:2923-6.
- [CrossRef] [PubMed] [Google Scholar]
- Hodgkin's lymphoma of the stomach: A rare entity. Indian J Med and Paediatr Oncol. 2019;40:427-9.
- [CrossRef] [Google Scholar]
- S3139 primary gastric Hodgkin's disease: A very rare entity. Am J Gastroenterol. 2021;116:S1294.
- [CrossRef] [Google Scholar]
- Classic Hodgkin lymphoma involving spleen, stomach, and pancreas without peripheral lymphadenopathy: Report of a very rare case. Libyan J Med Sci. 2022;6:23-6.
- [CrossRef] [Google Scholar]
- A rare case of primary gastric Hodgkin lymphoma in an adolescent with Nijmegen breakage syndrome. BMC Pediatr. 2023;23:189.
- [CrossRef] [PubMed] [Google Scholar]
- A case report of synchronous primary gastric Hodgkin lymphoma and lung adenocarcinoma and literature review. J Appl Hematol. 2023;14:62-6.
- [CrossRef] [Google Scholar]
- Primary gastric Hodgkin's lymphoma. World J Surg Oncol. 2007;5:119.
- [CrossRef] [PubMed] [Google Scholar]







