Translate this page into:
Evaluating the levels of D-dimer as a primary marker in oral cancers using immunoturbidimetric assay: The original research

*Corresponding author: Ashok K. Vikey, Department of Oral Pathology and Microbiology, Government College of Dentistry, Sardar Patel Marg, Indore - 452 001, Madhya Pradesh, India. drvikey73@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vikey AK, Parwani R, Asteker M, Gupta D, Parwani S. Evaluating the levels of D-dimer as a primary marker in oral cancers using immunoturbidimetric assay: The original research. Int J Mol Immuno Oncol 2020;5(3):96-100.
Abstract
Objectives:
Oral cancer is major health threat; with 90% mortality and ranks sixth among worldwide cancers. So to overcome this mortality; newer bio-markers are explored and one of such biomarker is D-dimer, which is end product of fibrinogen formed by plasmin. The raised levels of D-dimer play significant role in proliferation and progress of cancer cells. In cancers D-dimer is formed by dual action, where UPA (Urokinase type Plasminogen Activator) and Tissue factor play important role simultaneously. To understand correlation between D- dimer and oral cancers, by immunoturbidimetry; quantitative assay.
Material and Methods:
After obtaining consents of patients and Institutional ethical clearance, we randomly selected; age and sex matched; 216 samples. Further these samples were subdivides as oral cancer group and control group, consisting 108 samples in each group respectively.
Results:
Statistical analysis was done; using SPSS version 20, unpaired -T test, and one way ANOVA were applied. Plasma D-dimer levels were; 497.32±872.28μl/ml and 165.30±150.43 28μl/ml, among cancer and control groups respectively, (P≤ 0.0001), which was statistically highly significant.
Conclusion:
D-dimer is altered during carcinogenesis by activation of UPA and Tissue factors, and this distinguishes form routine levels of D-dimer. This suggests that, cancer cell biology is greatly affected by D-dimer levels during growth and spread of cancers. So raised levels of D-dimer can be considered during interventions of cancers, and incorporated as a biomarker. However for its scientific applications; there is need of further study, with collaborative approach and larger samples, to restrict cancer related mortalities.
Keywords
Oral cancer
D-dimer
Metastasis
Biomarker
INTRODUCTION
There is a long history of blood cells and growth of cancer, where angiogenic process plays crucial role in growth and proliferation of cancers. The processes called angiogenic switch, are a part of neovascularization against the stimulus by cancer cells from microenvironment or by precursors of bone marrow.[1,2]
The association between hematogenous factors and cancers was studied by Armand Trousseau, to explain alterations in blood leading to thrombophlebitis, with this, he introduced “Trousseau’s syndrome.”[3] Metastasis and its biological behavior were 1st time explained by Stephen Paget in 1889. He advocated importance of tumor microenvironment in metastasis and localization of tumors at different sites, later on termed as seed and soil theory. This theory is based on the growth of cancer cells (the seed), growing in suitable microenvironment at distant organ (the soil).[4] While exploring tumor microenvironment, researchers have identified many biological markers, and one of such markers is D-dimer. D-dimer is a cross-linked degradation product of fibrin, and their presence in human plasma is suggestive of fibrinolytic activity.[5,6]
Previously, D-dimer was known for activation of hemostasis and fibrinolysis, mainly in various systemic blood dyscrasias and other cancers of body.[7] The researchers have proved that the presence of D-dimer in plasma is challenging diagnostic marker for various pathological conditions and cancers, where exposure of monoclonal antibodies with fibrinogen contributes to proliferation and growth tumor.[8]
Nowadays, many tumor markers are available for screening, diagnosis, establishing prognosis, monitoring treatment, and detecting relapse. However, most tumor markers do not have sufficient sensitivity or specificity during screening and difficult to be detected in the early stages of malignancies.[9]
In this sequence of introducing new biomarkers, one of such feasible markers is D-dimer. This is accurate, rapid, and cost- effective marker, so gradually gaining entry as a biomarker, in the cancer interventions and monitoring systems.[10] By virtue of its feasibility, specificity, and cost-effectiveness, D-dimer is going to be an important tool during screening and management of all kinds of cancers.[11]
Oral cancer is a global health-care burden, with incidence rates of around 275,000/year, out of which two-third burden is on developing countries, mainly Southeast Asia, part of Europe, Latin America, and Pacific regions, with almost 3% of new cases every year. The records of 1990–1999 show that affected males doubled from 0.6 to 1.3/100,000 populations, with an average age of 4th and 6th decades, in developing and developed countries, respectively.[12]
Understanding the need, we selected this topic for study, considering that knowledge of this biomarker may explore newer avenues for favorable interventions of oral cancer patients.[7]
Aim and objectives
Aim
This study aims to understand the correlation between oral cancers and D-dimer, which is coagulation cascade component.
Objectives
The objectives of the study were as follows:
To assess the levels of D-dimer quantitatively in oral cancer patients.
To determine the levels of D-dimer in age- and sex- matched healthy controls.
To compare the coagulation assays between healthy controls and oral cancer patients.
MATERIALS AND METHODS
This is a case–control study, where plasma D-dimer levels are compared between two different groups, i.e., oral squamous cell carcinoma (OSCC) and controls. The duration of study was 2 years, March 2015–March 2017.
After obtaining institutional ethical clearance and written consents from all participants, recorded detailed case history and thorough clinical examination were done. A total of 216 samples were taken for the study, which further divided into two groups, such as Group A (oral cancers) and Group B (controls), with 108 samples in each group.
Inclusion criteria [Figures 1 and 2]
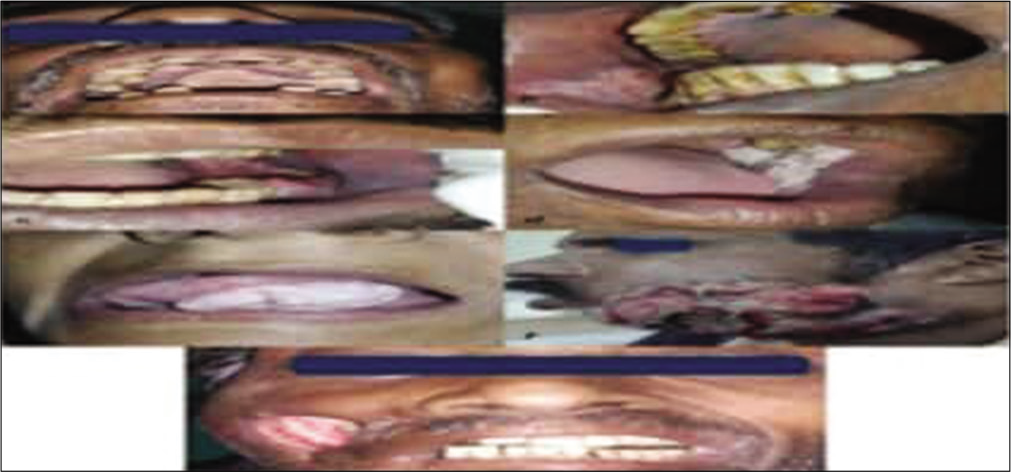
- Clinical photographs showing different growth patterns in oral cavity.
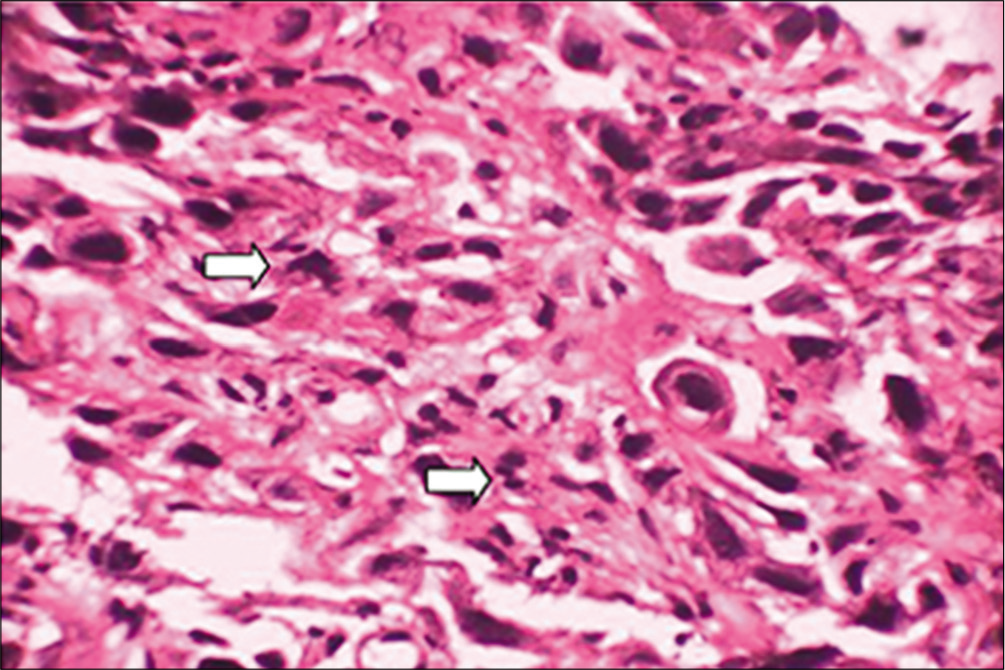
- Photomicrograph showing widespread dissociation of dysplastic epithelial cells with complete loss of intercellular adhesion, mitotic figures, and abnormal mitoses PDSC (×40) (arrows shows the mitotic figures and abnormal mitoses).
The following criteria were included in the study:
Age- and sex-matched random sample selection.
Clinically and histopathologically confirmed cases of OSCC.
Age group, between 25 and 70 years.
Exclusion criteria
Patients suffering from systemic diseases such as bleeding and clotting disorders, inflammation, scleroderma, cardiac and renal disorders, and deep vein thrombosis.
Pregnant or lactating women.
Patients on medications such as anticoagulants, hormones, or oral contraceptives.
Under all aseptic precautions, 2 ml of venous blood collected by venipuncture in a disposable vials containing 0.2 ml of sodium citrate. Blood samples were allowed to stand for 1 h at room temperature and then centrifuged at 3000 rpm for 3 min to separate the plasma. The plasma samples were stored at −70°C until they were quantified for D-dimer levels. Estimation of plasma D-dimer levels assessed using a diagnostic kit of cross-linked fibrin degradation products.
The test kit contents
Turbodyne D-dimer activation buffer (R1): Ready to use activation buffer reagent.
Turbodyne D-dimer latex reagent (R2): A uniform suspension of polystyrene latex particles coated with mouse monoclonal anti-D-dimer antibody. The cross- linked fibrin degradation products, D-Dimer and D-Dimer E and high-molecular-weight derivatives are all recognized by the latex reagent.
Turbodyne D-dimer smart card: With D-dimer master calibration curve.
The frozen specimens were thawed at 37°C and the plasma was centrifuged before testing. The smart card was inserted into the card reader slot of the Turbodyne SC Turbidity- Nephelometry analyzer.
Principle
This is a turbidimetric immunoassay for the determination of D-dimer and is based on the principle of agglutination reaction. The instrument uses dual technology optics and is based on turbidimetry-nephelometry principle. A light beam with optimum intensity (Io) is passed through the test cuvettes; as a result there is Ag-Ab reaction, as the reaction is over the light falls on the photodetector placed at 180° and 135°. Reduction in intensity of transmitted light (It) measured at 180°, which corresponds to the concentration of the electrolyte. The presence of D-dimer in the test specimen results in the formation of an insoluble complex resulting in an increase in turbidity, which is measured at wavelength of 650 nm. The increase in turbidity corresponds to the concentration of D-dimer in the test specimen.[13]
Test procedure
180 μl of the activated buffer (R1) and 20 μl of the sample mixed and incubated in the incubation chamber for 3–5 min.
Then, the cuvettes were placed in the reading chamber.
After that 60 μl of the latex reagent (R2), pipetted into the cuvette with the electronic pipette, the reaction started and the results were displayed on completion of the reaction.
D-dimer values analyzed statistically using SPSS version 20, where both descriptive and inferential statistics were used, applying unpaired T-test and one-way ANOVA. P < 0.05–P < 0.01 is considered as statistically significant, while from P < 0.009 to P < 0.001 is considered as statistically highly/strongly significant.
RESULTS
Immunoturbidimetry is one of the highly sensitive and specific and sensitive tests for D-dimer, rather superior to enzyme-linked immunosorbent assay (ELISA) and latex agglutination. According to our study, distribution of mean D-dimer levels among oral cancer cases showed raised levels as compare to healthy controls, with highly significant statistical difference (P < 0.0001) [Table 1 and Graph 1]. However, our other parameters, i.e., association between age and D-dimer, where 46.8% of subjects were between 31 and 45 years, 31.94% were 46 and 60 years, 15.76% in the range of 15 and 30 years, and 5.55% were more than 60 years, and there was no association between age and D-dimer values, P = 0.096 [Table 2 and Graph 2].
| Group | n | Mean D-dimer level±Std. deviation | t-value | Pvalue |
|---|---|---|---|---|
| Oral cancer | 108 | 497.3238±872.28013 | 3.898 | <0.0001* |
| Control | 108 | 165.3078±150.43115 |
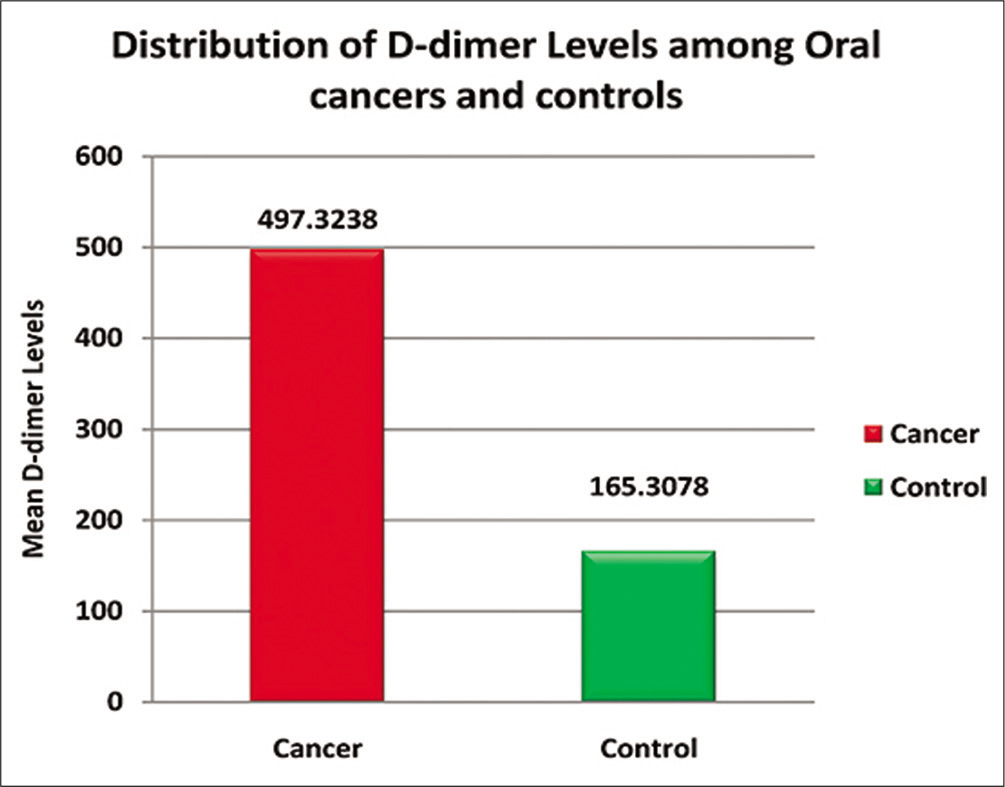
- Bar diagram showing the overall distribution of D-dimer levels (μl/ml) among oral cancers and controls.
| n | Mean D-dimer level±Std. deviation | F value | Pvalue | |
|---|---|---|---|---|
| 25–30 years | 34 | 140.2468±114.40212 | ||
| 31–45 years | 101 | 387.1290±792.57432 | 2.141 | 0.096 |
| 46–60 years | 69 | 293.8784±526.17390 | ||
| More than 60 years | 12 | 618.1817±679.00988 | ||
| Total | 216 | 331.3158±646.23202 |
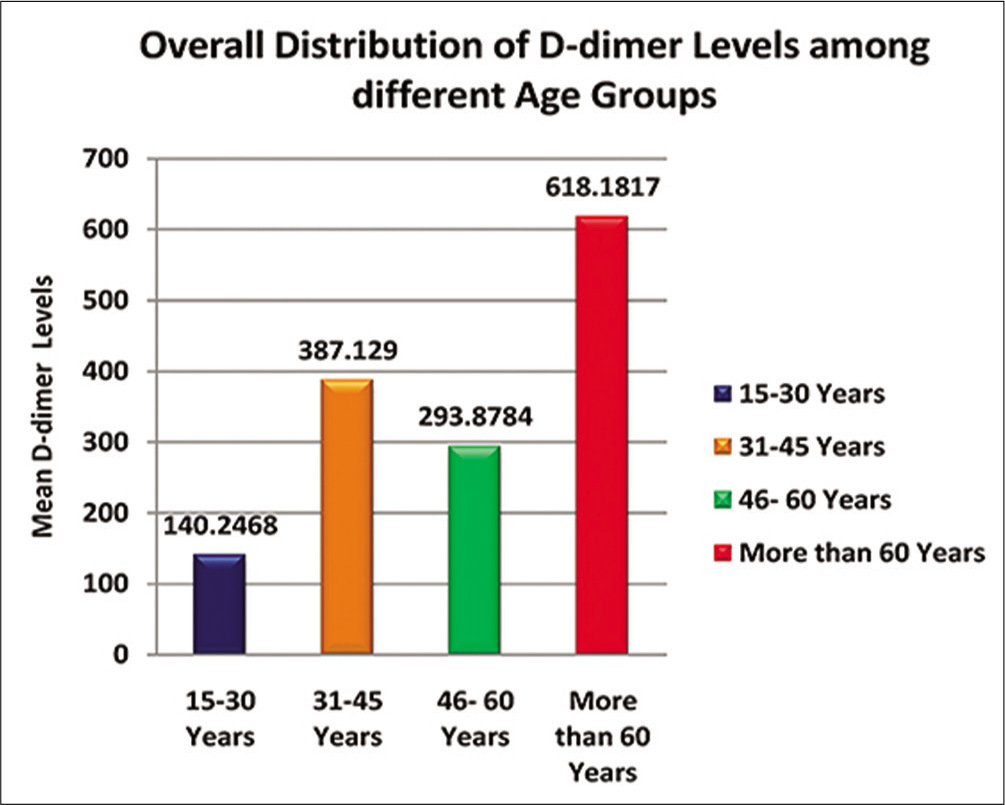
- Bar diagram showing the overall distribution of D-dimer levels among different age groups (μl/ml).
DISCUSSION
The nature of fibrinogen and its end products in routine physiology are to help in coagulation, repair of vessels, migration and proliferation of cells, angiogenesis, wound healing, and vascular contraction and relaxation. These properties can act as helpers when the cancer cells hijack the stromal components, leading to imbalance the hemostasis, by extra venous proteolysis and growth of transformed cancerous cells.[14] This concept is explained and highlighted in many studies, conducted on cancers of other body parts, which showed adverse post-treatment effects in cases with raised levels of plasma D-dimer, mainly during metastasis or nodal involvement.[15] To assess D-dimer levels, there are many techniques such as ELISA and latex agglutination, but we performed our study using one of the recent method, known as immunoturbidimetry, which is more specific and sensitive than other two techniques.
As per routine protocol of scientific studies guideline, which includes ethical clearance and consents, we took blood samples of participating subjects with minimal invasion.
Further, the samples were prepared to run on automatic Turbodyne analyzer and a company recruited skilled person assisted entire procedure. The reason behind this was to rule out even minor mistakes during procedure, so as to achieve best possible results. Being highly specialized technique, even minor errors may reflect the values of D-dimer. The results showed; comparatively raised levels of D-dimer in cancer population than controls, and these were statistically significant, P < 0.0001 [Table 1 and Graph 1].
The studies support our hypothesis, where hypercoagulability and fibrinolytic aspect, i.e., raised levels of D-dimer as a biomarker, has authentic role in many types of solid tumors and this affects interventions and prognosis.[16,17] There are many physiological interactions between microcellular and endothelial levels during tumorigenesis, which is effective in growth and progress of cancer cells. These changes affect extrinsic coagulation system to release D-dimer, further leading to fibrinolytic cascade within a tumor, for successful growth, invasion, and metastasis.[18] The raised levels of D-dimer in oral cancer patients and their post-treatment outcomes in our study are in accordance with the study of Oya et al., which showed higher D-dimer levels and their clinical outcome.[19,20]
We also assessed role of age factor and its relevance with D-dimer levels if any, but did not show any statistical significance P = 0.096 [Table 2 and Graph 2]. The scheduled post-operative follow-up at interval of 1 month was planned, and out of 108, 46 patients reported. The overall duration of follow-up was around 6 months, where we assessed post- operative healing, pain, jaw mobility, eating habit, saliva, teeth, and surrounding structures, where we noticed positive improvements and found positive correlation with D-dimer levels.
CONCLUSION
In this research, D-dimer levels were assessed quantitatively, by immunoturbidimetric assay, which is most authentic and superior analysis as compare to other methods such as latex agglutination, ELISA, and other qualitative tests. According to our knowledge, this is the first quantitative D-dimer analysis done with immunoturbidimetric method, which was conducted on oral cancer patients. Strict protocol was followed, under the supervision of skilled scientist throughout this study. With this high precision, we got highly significant results when compared, between oral cancer patients and healthy control group. These findings suggest that D-dimer can be effective biomarker during interventions of oral cancer patients to enhance survival of these patients. However, for further authenticity, it needs large sample size and longer duration with post-operative analysis of D-dimer.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Impaired recruitment of bone-marrow derived endothelial and hematopoietic precursor precursors blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194-201.
- [Google Scholar]
- Malignancy, thrombosis and trousseau: The case for an eponym. J Thromb Haemost. 2003;1:2463-5.
- [Google Scholar]
- Cancer metastasis: A product of tumor-host interactions. Curr Probl Cancer. 1979;3:1-59.
- [Google Scholar]
- Pro-metastatic tumor-stroma interactions in breast cancer. Future Oncol. 2012;8:1427-42.
- [Google Scholar]
- Association between plasma levels of D-dimer and fibrinogen/fibrin degradation products (FDP) for exclusion of thromboembolic disorders. J Thromb Thrombolysis. 2006;21:199-202.
- [Google Scholar]
- Evaluation of an automated, latex-enhanced turbidimetric D-dimer test (advanced D-dimer) and usefulness in the exclusion of acute thromboembolic disease. Am J Clin Pathol. 2003;120:930-7.
- [Google Scholar]
- High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97:1158-64.
- [Google Scholar]
- An overview of coagulation disorders in cancer patients. Surg Oncol. 2010;19:e33-46.
- [Google Scholar]
- Quantitative estimation of serum fibrinogen degradation product levels in oral premalignant and malignant lesions. J Int Oral Health. 2013;5:65-72.
- [Google Scholar]
- Coagulation tests show significant differences in patients with breast cancer. Tumor Biol. 2014;35:5985-92.
- [Google Scholar]
- D-Dimer: Not just an indicator of venous thrombosis but a predictor of asymptomatic hematogenous metastasis in gastric cancer patients. PLoS One. 2014;9:e101125.
- [Google Scholar]
- D-dimer levels and risk of recurrent venous thromboembolism. JAMA. 2003;290:1071-4.
- [Google Scholar]
- Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol. 2000;18:600-8.
- [Google Scholar]
- The role of D-dimer and residual venous obstruction in recurrence of venous thromboembolism after anticoagulation withdrawal in cancer patients. Haematologica. 2005;90:713-5.
- [Google Scholar]
- The role of fibrinogen, fibrin and fibrin (ogen) degradation products (FDPs) in tumor progression. Contemp Oncol (Pozn). 2013;17:113-9.
- [Google Scholar]
- D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol. 2010;21:1267-72.
- [Google Scholar]
- High preoperative plasma d-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31:388-94.
- [Google Scholar]
- Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br J Cancer. 2002;86:389-95.
- [Google Scholar]






