Translate this page into:
Complete metabolic response with letrozole and palbociclib in advanced breast cancer

*Corresponding author: Manikandan Dhanushkodi, MD DM DNB, Department of Medical Oncology, Cancer Institute (WIA), 38, Sardar Patel Road, Chennai - 600036, Tamil Nadu, India. dmani1982@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dhanushkodi M, Ananthi B, Iyer P. Complete metabolic response with letrozole and palbociclib in advanced breast cancer. Int J Mol Immuno Oncol 2019;4(3):82-4.
Abstract
We report a patient with hormone positive, HER2 negative advanced breast cancer with extensive metastasis who achieved complete metabolic remission with letrozole and palbociclib
Keywords
Advanced breast cancer
Palbociclib
Complete metabolic remission
INTRODUCTION
A 60-year-old woman presented with a lump in the right breast and headache of 1 month duration. On examination, she had a 4 × 4 cm mass in the right breast and right axillary lymph node of size 1.5 cm. Biopsy from the right breast mass was suggestive of infiltrating ductal carcinoma, Grade 3, immunohistochemistry showed estrogen receptor (ER) positivity, progesterone receptor positivity, and HER2 (2+). FISH for HER2 neu was negative. FDG-PET/ CT (18-fluorodeoxyglucose-positron emission tomography/computed tomography) showed extensive disease with uptake in the right breast, lymph nodes in bilateral axilla, supraclavicular region, mediastinum, retroperitoneal, liver, brain, lytic lesions in the skull, bilateral humerus, ribs, sternum, scapulae, clavicle, vertebrae, sacrum, and pelvic bones [Figure 1]. She underwent palliative whole brain radiation 30 Gy and was planned for capecitabine (1 g/m2/day in two divided doses for 14/21 days) and zoledronic acid (4 mg Q 3 monthly). The patient stopped capecitabine after 1 week in view of fatigue, gastritis, and diarrhea. She was switched to letrozole (2.5 mg daily) and palbociclib (125 mg daily for 21/28 days). She tolerated this regimen well without any side effects including neutropenia. After 6 months of therapy, she had an ill-defined residue in the upper inner quadrant of the right breast. PET-CT showed a complete metabolic remission with sclerosis of bone lesions with no standardized uptake value, Figure 2. She was symptom free for 1 year after which she had altered sensorium and expired probably due to progressive central nervous system (CNS) disease.
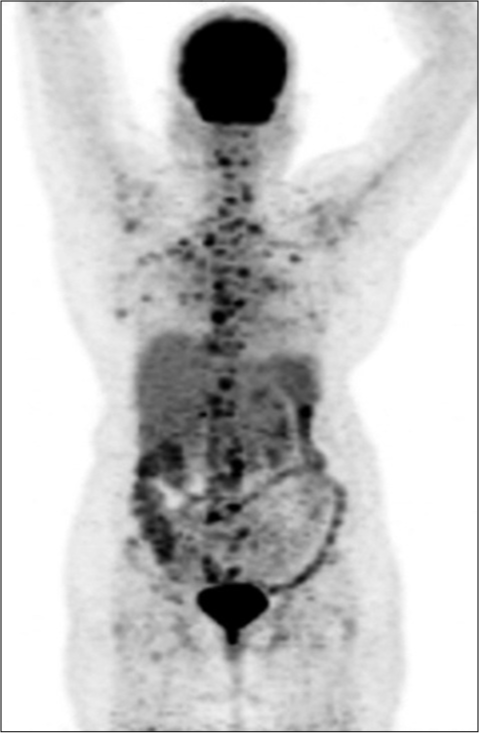
- Baseline PET CT showed extensive disease with uptake in breast, nodes, bones.
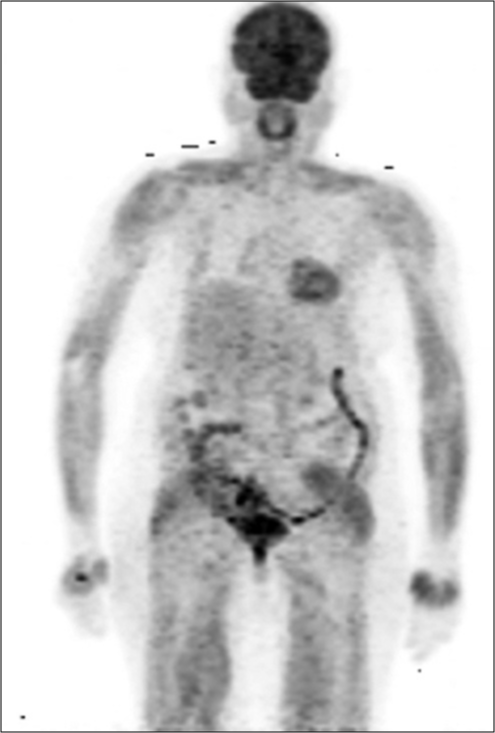
- PET CT after 6 months of letrozole and palbociclib showing showing complete metabolic remission.
DISCUSSION
Palbociclib is a CDK 4/6 inhibitor approved for use in combination with letrozole in patients with ER-positive and ERBB2-negative advanced breast cancer.[1] The other CDK 4/6 inhibitors including ribociclib[2] and abemaciclib[3] in combination with letrozole have also shown significant improvement in progression-free survival as compared to letrozole alone. The advantages of CDK 4/6 inhibitors are the oral schedule and minimal toxicity.[4] The objective response rates of palbociclib, ribociclib, and abemaciclib in combination with letrozole were 42%, 40%,and 35%, respectively. However, complete remission was uncommon in these trials. We would like to record the observation of a patient with Stage IV breast cancer with extensive metastatic disease involving bones, nodes, liver, and brain achieving complete metabolic remission with letrozole and palbociclib. There is one published report of a patient with liver and nodal metastasis who achieved complete metabolic response.
The clinical trials with CDK4 inhibitors have used CT scan, magnetic resonance imaging, and bone scintigraphy for reassessment (RECIST version 1.1), but we assessed the patient with FDG-PET/CT scan. The metabolic information from FDG-PET/CT is more sensitive than conventional imaging for distant metastasis[5] and better for monitoring response to therapy.[6,7] Complete remissions in breast cancer are inversely proportional to the initial tumor bulk[8] and this patient achieved metabolic remission although she had an extensive tumor burden. PET-CT response correlates with pathologic complete response in patients with HER2- positive breast cancer treated with neoadjuvant therapy with trastuzumab and pertuzumab.[9]
CDK 4/6 studies with palbociclib[1] and ribociclib[10] have excluded patients with brain metastasis at presentation. As of now, there are no published studies with palbociclib-induced CNS response. One ongoing study is testing the role of palbociclib in HER2-positive breast with brain metastasis.[11] Our patient had complete metabolic remission in spite of having brain metastasis at presentation.
At present, there is no standard of care for patients who progress after hormonal therapy with CDK4/6 inhibitor. The treatment options include fulvestrant, everolimus- exemestane, and chemotherapy. Whole-exome sequencing of the tumor would be valuable in ascertaining whether there was any genetic reason for this exceptional response.
CONCLUSION
Letrozole and palbociclib can cause complete metabolic response in hormone-positive and HER2-negative advanced breast cancer.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925-36.
- [CrossRef] [PubMed] [Google Scholar]
- Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-48.
- [CrossRef] [PubMed] [Google Scholar]
- MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638-46.
- [CrossRef] [PubMed] [Google Scholar]
- Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res. Treat. 2017;166:41-54.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent and metastatic breast cancer PET, PET/ CT, PET/MRI: FDG and new biomarkers. Q J Nucl Med Mol Imaging. 2013;57:352-66.
- [Google Scholar]
- The role of PET/CT for evaluating breast cancer. Korean J Radiol. 2007;8:429-37.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnostic value of PET/CT in recurrence and distant metastasis in breast cancer patients and impact on disease free survival. Egypt J Radiol Nucl Med. 2014;45:1317-24.
- [CrossRef] [Google Scholar]
- Complete remissions in metastatic breast cancer treated with combination drug therapy. Ann Intern Med. 1979;91:847-52.
- [CrossRef] [PubMed] [Google Scholar]
- TBCRC026: Phase II trial correlating standardized uptake value with pathologic complete response to pertuzumab and trastuzumab in breast cancer. J Clin Oncol. 2018;37:714-22.
- [CrossRef] [PubMed] [Google Scholar]
- Ribociclib as first-line therapy for hr-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-48.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study of palbociclib in patients with HER2-positive breast cancer with brain metastasis. J Clin Oncol. 2017;35
- [CrossRef] [Google Scholar]






