Translate this page into:
Cardiotoxicity in patients on trastuzumab in HER2-positive breast cancer – A retrospective analysis from a center in North India

*Corresponding author: Anjali Aggarwal, Department of Research, Max Superspeciality Hospital, Shalimar Bagh, Delhi - 110 088, India. anjaliagg412@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Aggarwal A, Patil P, Rangaraju RR, Abbas W, Garg S. Cardiotoxicity in patients on trastuzumab in HER2-positive breast cancer – A retrospective analysis from a center in North India. Int J Mol Immuno Oncol 2021;6(2):56-60.
Abstract
Objectives:
Cardiotoxicity has been associated with trastuzumab for long and its relation with anthracyclines has also been well established. The study aims to assess the cardiotoxicity in patients on trastuzumab in human epidermal growth receptor 2-positive breast cancer.
Material and Methods:
This retrospective study consisting of a 3 years database of 112 patients with breast cancer from a tertiary care center in India. A total of 64 patients were scrutinized meeting the eligibility criteria. The primary eligibility criteria were availability of baseline, 3 monthly, end of treatment, and 3 months post-treatment left ventricular ejection fraction (LVEF) profile data.
Results:
41/62 patients (66.1%) showed decrease in the LVEF profiles having mean reduction of 6%. Of these 41 patients, 34 (53.1%) patients exhibited a drop of 0–5%, 4 (6.2%) showed a drop of 6–10%, and 3 (4.6%) patients showed a drop of more than 10%. A significant drop (more than 10%) in the LVEF profile was observed in mean time of 6 months (8 cycles) which recovered to baseline value with the median follow-up of 3 months post-cessation of trastuzumab. Most of patients in the LVEF drop >10% group (70%) had received an anthracyclines based regimen.
Conclusion:
Our study demonstrated a mean drop of >10% in the LVEF profiles (4.6%) patients after mean follow-up of 6 months after starting therapy with trastuzumab and reverted back to baseline value after over a mean of 3 months post completing therapy with trastuzumab. This warrants regular and strict surveillance for the first 6 months and thereafter also after starting therapy with trastuzumab.
Keywords
Cardiotoxicity
Trastuzumab
Human epidermal growth factor 2
Positive breast cancer
Left ventricular ejection fraction
INTRODUCTION
Breast cancer is the second most common cancer worldwide and the most common in women as per worldwide data till 2018 with an incidence of 2.08 million new cancer.[1] Breast cancer is the most common cause of death in women worldwide and accounts for about 522,000 thousand deaths becoming the second most common cause of death accounts for about 324,000 deaths in more developed regions after the lung cancer. In India, there is an increase in incidence and mortality by 11.54% and 13.82%, respectively, during the years 2012–2018.[2] Breast cancer is a heterogeneous disease,[3] categorized into different molecular subtypes based on the genetic makeup.[4]
Trastuzumab has for long been associated with reversible cardiotoxicity in the historical trials.[5,6] The first unanticipated adverse effect was recognized from the early trials in metastatic Her2-positive breast cancer.[7-11] Trastuzumab is a humanized monoclonal antibody that acts by inhibiting the extracellular domain of the tyrosine kinase receptor.[12] The main adverse event associated with trastuzumab is cardiotoxicity seen in about 2–7% of patients.[13] The congestive heart failure adverse effect that has been of concern has been under surveillance and monitoring. The data from multiple Phase III trials have shown that there is a significant decline in the left ventricular ejection fraction (LVEF) profiles, which has guided us in deciding treatment in this subset of patients. As per the guidelines, close monitoring is necessary for every 3 months to predict the cardiotoxicity in these patients on adjuvant treatment with trastuzumab. This real-time study of Indian patients from North India will help us in further standardizing the care and utility of monitoring the echocardiogram (ECHO) profiles and predicting the management of the patients with the LVEF decline. The aim is to evaluate the cardiotoxicity in patients receiving trastuzumab in human epidermal growth receptor 2 (HER2) breast cancer.
MATERIAL AND METHODS
This was a retrospective study consisting of a 3 years database of 112 patients with breast cancer from a tertiary care center in India from 2015 to 2018. A total of 64 patients were scrutinized after exclusion criteria depending on completion of treatment outpatient department visits, inadequate ECHO profile or performed at a different center or by a different cardiologist and brand of trastuzumab used. The patient data were collected retrospectively from database using system captured data and files of patients more than 18 years of age with breast cancer on trastuzumab in the curative setting, either neoadjuvant or adjuvant. Trastuzumab was given for a total of 12 months in all patients, with a loading dose of 8 mg/kg in the first cycle followed by 6 mg/kg from the second cycle till the last. The brand of trastuzumab used was similar in all the patients. The primary eligibility criteria were the availability of baseline, 3 monthly, and end of treatment till 3 months post-treatment LVEF profile data. The method used for the determination of EF by ECHO was Simpson’s formula in all the patients. ECHO profile was done till 6 months post completing therapy with trastuzumab. The statistical software used for analysis was SPSS version 2.0.
RESULTS
This retrospective study analyzed a pool of 112 patients. Out of 112 patients, 64 were analyzed depending on the eligibility for the assessment of data. The drop in the analysis of the potential pool of 112 patients to 64 patients was because of the failure of meeting the primary eligibility criteria of having the record of either one ECHO profile at the baseline and one at the end of the treatment or ECHO performed by a different cardiologist or at a different center. The subjects not completing these primary eligibility criteria were not taken into the account. The results were out of 41 (66.1%) out of 64 patients showed a decrease in the LVEF profiles having a mean reduction of 6%. The total pool of patients that showed a drop of >10% came out to be 4.3% consistent with the other historical studies[14] [Table 1 and Figure 1].
| LVEF profile | Number of patients n=41 | % |
|---|---|---|
| Patients dropped LVEF 0–5% | 34 | 53.1 |
| Patients dropped LVEF 6–10% | 4 | 6.2 |
| Patients dropped LVEF more than 10% | 3 | 4.6 |
LVEF: Left ventricular ejection fraction

- Patients with reduced left ventricular ejection fraction profiles.
Out of these 41 patients evaluated, 34 (53.1%) patients demonstrated a drop of 0–5%, 4 patients (6.2%) between 6 and 10%, and 3 patients (4.6%) more than 10%. Furthermore, the results from the analysis were evaluated based on the type of regimen received. Of the 64 analyzed, 42 patients received the taxane-carboplatin-trastuzumab regimen, 14 received anthracyline-cyclophosphamide regimen followed by taxane-trastuzumab, and 8 patients received non-anthracyclines-trastuzumab based regimen. Furthermore, of the 64 analyzed, 22 (34%) patients were left-sided breast cancers that had received radiation therapy.
The drop in the LVEF profiles as shown in Table 2 shows out of 34 patients in Group 1 (LVEF drop 0–5%), 29 received anthracycline-trastuzumab regimen, and 5 patients received non-anthracycline-trastuzumab regimen. In Group 2 (LVEF drop 6–10%) of four patients, three received anthracycline-trastuzumab regimen and one patient received non-anthracycline-trastuzumab regimen. In Group 3 (LVEF drop >10%) of three patients, two received anthracycline-trastuzumab regimen and one patient received non-anthracycline-trastuzumab regimen. P = 3.33 was not statistically significant [Table 2 and Figure 2].
| Treatment drop (n=41) | AC-TH (n=14) | TCH (n=42) | Non-anthracycline (n=8) | Pvalue |
|---|---|---|---|---|
| LVEF (0–5%) (n=34) | (90%) 9 | (83.3%) 20 | (71.4%) 5 | 0.33 |
| LVEF (6–10%) (n=4) | 0 | (12.5%) 3 | (14.2%) 1 | |
| LVEF >10% (n=3) | (10%) 1 | (4.1%) 1 | (14.2%) 1 |
LVEF: Left ventricular ejection fraction
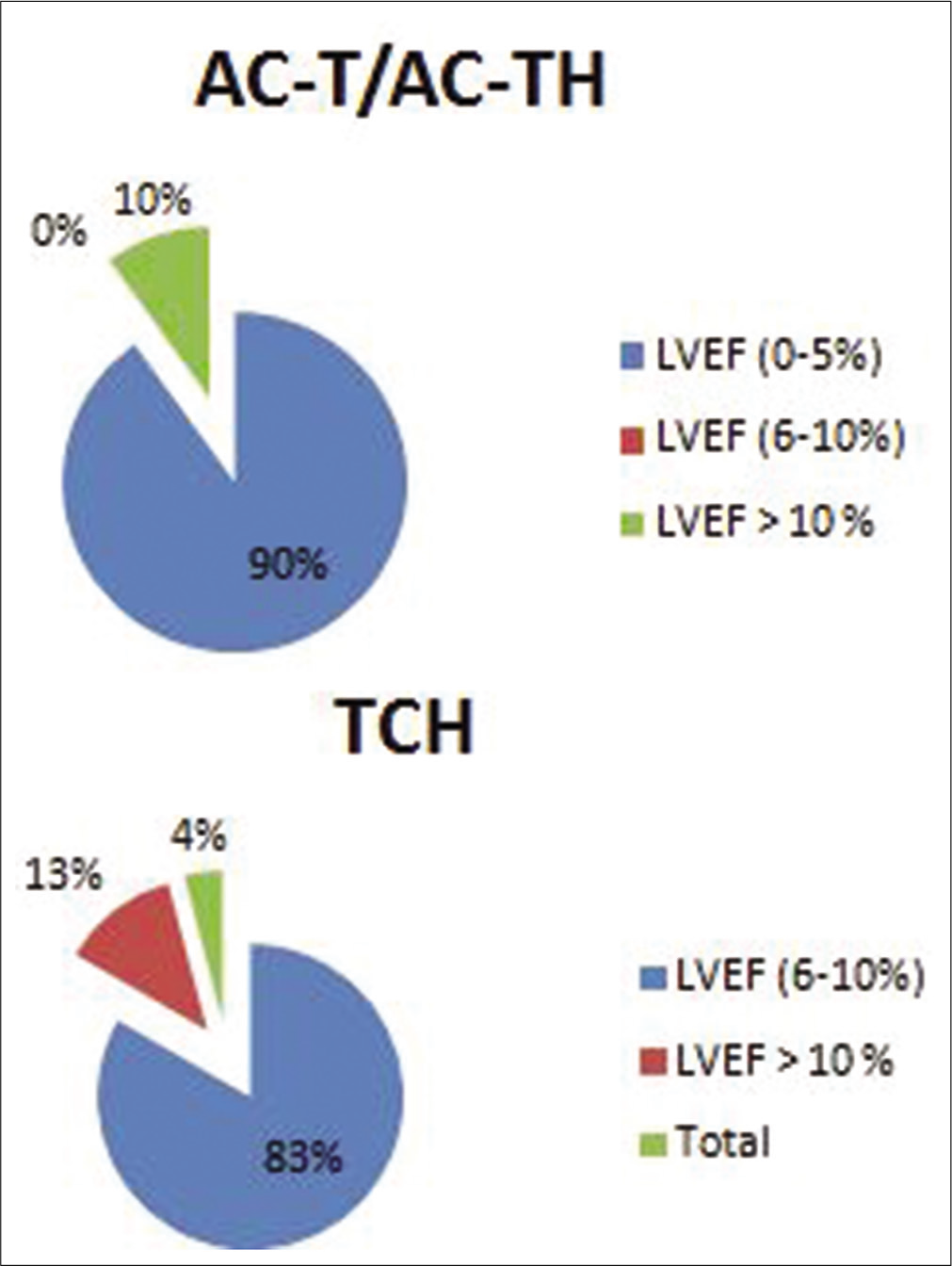
- Left ventricular ejection fraction profiles in different regimens.
Of the 41 patients with reduced LVEF, 14 patients (34%) were left-sided breast cancers that had received radiation therapy to ipsilateral breast and axilla. As evaluated on the basis of pre-existing comorbidities, the maximum no. of patients had a drop in the LVEF profile of 0–5% group in hypertension/ coronary artery disease/diabetes mellitus, which was 80.6% followed by 88.8% in group having no comorbidities and 50% in the group having other comorbidities. Out of 14 patients receiving concurrent cardio protective medication (beta blockers, ACE inhibitors, a drop of LVEF profile 0-5% were seen in 8 subjects (88%) and 1(11%) subjcet had a drop of >10% of total 14 subjects who were having comorbidities. In the group of patients having no comorbidities, the LVEF drop was 12.1% in the LVEF 6–10% drop group and 6.4% in the LVEF drop >10% group. The power of significance calculated by the Chi-square test of independence was <0.05 [Table 3 and Figure 3].
| LVEF (n=64) | 0–5% | 6–10% | >10% | Pvalue |
|---|---|---|---|---|
| n (n=48) | 25 (80.6) | 4 (12.1) | 2 (6.4) | <0.05 |
| HTN/DM/CAD (n=14) | 8 (88.8) | 0 | 1 (11.1) | |
| Others (n=2) | 1 (50) | 0 | 0 |
LVEF: Left ventricular ejection fraction, HTN: Hypertension, CAD: Coronary artery disease, DM: Diabetes mellitus
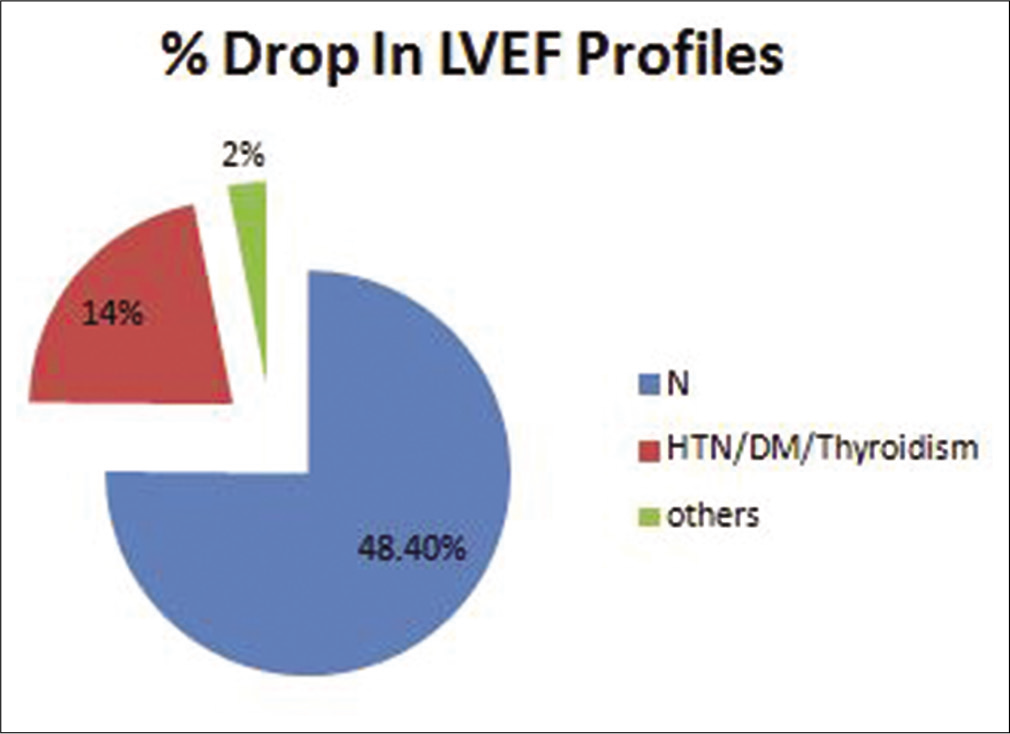
- Left ventricular ejection fraction profile in different comorbid conditions.
DISCUSSION
In this retrospective analysis, the trastuzumab-induced cardiotoxicity was monitored using echocardiography. Troponins I and T (TnI and TnT, both markers of myocardial injury), B-natriuretic peptide (BNP a marker of wall stress), and C-reactive protein (CRP; a marker of inflammation) are the several other different potential biomarkers which have been studied for the same.[15]
TnI positivity has been proven to be beneficial in identifying patients who are at risk for trastuzumab-induced cardiotoxicity unlikely to recover from cardiac dysfunction despite HF therapy.[16] Another study analyzed eight different parameters as a potential biomarkers for trastuzumab-related cardiotoxicity.[17] These parameters were ultrasensitive TnI, high sensitivity CRP, N-terminal pro-B-type natriuretic peptide, growth differentiation factor-15, myeloperoxidase (MPO), placental growth factor, soluble fms-like tyrosine kinase receptor-1, and galectin-3. This study revealed that early increases in TnI and MPO levels offer additive information about the risk of cardiotoxicity in patients undergoing doxorubicin and trastuzumab therapy. In another pilot study by Onitilo et al.,[18] patients with normal hs-CRP had low risk of developing trastuzumab-related decreased LVEF. However, no such association with seen with BNP or cTnI. As of now, there are no definite recommendations for routine use of biomarkers in predicting trastuzumab-related cardiotoxicity.[19] Current standard of practice for the evaluation of trastuzumab-related cardiotoxicity is echocardiography and MUGA. The latter one is less preferred because of the injectable tracer and economical constraints.[15]
In our study, the percentage of patients having a significant drop of LVEF was 3/62 (4.6%). Out of these three patients, one patient had a drop in LVEF profile by 17% from 62% to 45%, whereas the other two patients LVEF dropped by 12% and 14%, respectively. They were then taken off from the trastuzumab therapy and closely monitored for further drop; meanwhile, the treatment was put on hold and ECHO profiles were monitored every monthly till regaining of baseline LVEF profile. The median LVEF profiles maintained at the baseline in 0–5% group were 52.7 and dropped to a mean LVEF of 51.5. In patients, whose LVEF dropped from 6% to 10% group maintained at baseline was 65.25 and dropped to 56.75. In the third group of patients, whose LVEF profile dropped to >10% was maintained at 62.6 and dropped to mean value of 48.3. The mean follow-up of these patients was 10.5 months.
The LVEF profile drop on trastuzumab therapy >10% was observed only in 4.6% of the patients and the use of life-saving drug should not be dropped in the patients having normal LVEF profiles. The significant drop seen in this study is consistent with the available historical data.[14] In this real-time data, no patient whose cardiac profile was below the normal was prone to develop cardiotoxicity and the significant number of patients did not tend to develop symptomatic heart failure while on the treatment. In clinical trials, the patients developing symptomatic HF during trastuzumab has been relatively low (0.6–3.8%) which has not reflected in our data possibly because of less number of patients evaluated as compared to historical trials.[20-24] In the view of the safety profiles, data from studies with greater follow-up are required.
In the first few months of initiating therapy with trastuzumab for patients with HER2-positive breast cancer, discontinuing the medication is based on solely symptoms of cardiac failure, than monitoring the LVEF profiles. This has made real-world data very rigid to analyze. It is important to realize for physicians that the patient pool eligible for treatment with trastuzumab is much less as compared to the patient pool of data trials, so monitoring of these patients is important.
A holistic approach is required to study the safety of trastuzumab in these subsets of patients who are prone to develop cardiotoxicity and will help to shape the monitoring schedules. Furthermore, a multidisciplinary team consisting of oncologists, cardiologists, and supportive medical staff is required to decide and monitor the LVEF and clinical profile of those patients. Newer modalities like three-dimensional Echoes, Cardiac MRIs and specific biomarkers should be used in the future for risk stratification to improve outcomes.
CONCLUSION
Our study demonstrated a mean drop of >10% in LVEF profiles in 4.6% of patients which developed over after mean of 6 months after starting therapy with trastuzumab and reverted back to baseline value after over a mean of 3 months post-stopping therapy with trastuzumab which warrants regular and strict surveillance for the first 6 months and thereafter also after starting therapy with trastuzumab. Furthermore, the identification of patient subset for developing clinical cardiac failure should be identified with performing baseline Echo testing and monitored thereafter in regular successions.
Declaration of patient consent
In the view of the retrospective nature of the study, a waiver of consent was obtained.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Integrative clustering reveals a novel split in the luminal a subtype of breast cancer with impact on outcome. Breast Cancer Res. 2017;19:44.
- [CrossRef] [PubMed] [Google Scholar]
- Globocan 2018-Breast Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2018.
- [Google Scholar]
- Epidemiology of breast cancer in Indian women. Asia Pac J Clin Oncol. 2017;13:289-95.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac monitoring during adjuvant trastuzumab-based chemotherapy among older patients with breast cancer. J Clin Oncol. 2015;33:2176-83.
- [CrossRef] [PubMed] [Google Scholar]
- Breast cancer subtypes: Morphologic and biologic characterization. Womens Health (Lond). 2016;12:103-19.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiotoxicity and cardiac monitoring during adjuvant trastuzumab in daily Dutch practice: A study of the Southeast Netherlands breast cancer consortium. Oncologist. 2016;5:555-62.
- [CrossRef] [PubMed] [Google Scholar]
- Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859-65.
- [CrossRef] [PubMed] [Google Scholar]
- Trastuzumab in female breast cancer patients with reduced left ventricular ejection fraction. J Am Heart Assoc. 2018;7:e008637.
- [CrossRef] [PubMed] [Google Scholar]
- Trastuzumab-induced cardiotoxicity in early breast cancer patients: A retrospective study of possible risk and protective factors. Heart. 2013;99:634-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015;12:547-58.
- [CrossRef] [PubMed] [Google Scholar]
- Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-84.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215-21.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236-44.
- [CrossRef] [Google Scholar]
- Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA Oncol. 2016;2:29-36.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term cardiac safety analysis of NCCTG N9831 (alliance) adjuvant trastuzumab trial. J Clin Oncol. 2016;34:581-7.
- [CrossRef] [PubMed] [Google Scholar]
- Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820-6.
- [CrossRef] [PubMed] [Google Scholar]
- Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J Clin Oncol. 2005;23:2900-2.
- [CrossRef] [PubMed] [Google Scholar]
- Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open. 2016;1:e000073.
- [CrossRef] [PubMed] [Google Scholar]
- Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910-6.
- [CrossRef] [PubMed] [Google Scholar]
- Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809-16.
- [CrossRef] [PubMed] [Google Scholar]
- Trastuzumab-induced cardiotoxicity: Is it time for troponin for all patients? Am J Clin Oncol. 2012;35:183-4.
- [CrossRef] [PubMed] [Google Scholar]
- High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: A pilot study. Breast Cancer Res Treat. 2012;134:291-8.
- [CrossRef] [PubMed] [Google Scholar]






