Translate this page into:
Breast carcinoma disguised as systemic sclerosis: A case report of unusual paraneoplastic syndrome

*Corresponding author: Alfred Patrick Dionisio Mina, Section of Medical Oncology, The Medical City, Ortigas Avenue, Pasig City, Philippines. alfredpatrickmina@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Mina APD, Bonci EA, Louro T. Breast carcinoma disguised as systemic sclerosis: A case report of unusual paraneoplastic syndrome. Int J Mol Immuno Oncol. 2025;10:37-40. doi: 10.25259/IJMIO_4_2024
Abstract
A 70-year-old female initially presented in the clinic for a history of skin thickening, fatigue, weight loss, shortness of breath, and Raynaud’s phenomenon. She consulted an immunologist, and the workup revealed a positive test for antinuclear antibody and anti-polymerase III antibody. A diagnosis of systemic sclerosis (SSc) was made due to the presence of a non-specific interstitial change pattern on the thoracic computed tomography scan. The patient was initially treated with steroids and non-steroidal anti-inflammatory drugs. However, the disease progressed, and treatment was shifted to mycophenolate mofetil and nintedanib. Two years after the diagnosis, she developed five liver nodules, which were positive for carcinoma of breast cancer origin, hence the diagnosis of metastatic breast carcinoma. The first line of treatment with ribociclib and letrozole was initiated. Six months after the oncologic treatment initiation, there was a significant clinical improvement in the symptoms, partial response of the liver nodules with no metabolic expression, and stable lung interstitial changes. At present, the patient continues her breast cancer and SSc maintenance treatment. The lack of response to conventional SSc therapy but improvement with the underlying malignancy treatment suggests the case’s possible paraneoplastic origin. This case report may further improve awareness of the natural course of the disease, preventing a potential cancer treatment delay.
Keywords
Anti-polymerase III antibody
Breast cancer
Paraneoplastic syndrome
Systemic sclerosis
INTRODUCTION
Systemic sclerosis (SSc) or scleroderma is a connective tissue disease, usually of unknown origin and has unpredictable course.[1] The clinical manifestation is highly heterogeneous and affects multiple vascular beds, characterized by autoimmunity and inflammation with widespread vasculopathy. The systemic progression is linked to extensive tissue fibrosis. Several papers pointed out T cells’ role in the SSc pathogenesis. An increased number of T cells and their markers were found in patients with SSc peripheral blood.[2]
Autoimmune paraneoplastic syndromes may also occur in breast cancer. Polymyositis/dermatomyositis (DM) is common, but SSc may also occur.[3] There are reports of paraneoplastic SSc occurring as a discrete manifestation secondary to an underlying malignancy. Often, it is difficult to distinguish if the SSc occurs as a paraneoplastic syndrome from a masked malignancy or if the SSc resulted in cancer as the disease activity and treatment are considered risk factors. Different influences such as age at diagnosis, presence of distinct antibodies, treatment history, previous rheumatic conditions, and temporal pattern may help establish the correct diagnosis. It is noteworthy to distinguish that those SSc resolving with anti-cancer therapy raises the possibility of paraneoplastic syndrome. The case report that we will present sheds an understanding of the behavior of cancer as its initial SSc presentation was negative for malignancy and, later on, improved to cancer treatment when a distal malignancy was discovered on re-investigation.
CASE REPORT
This is a case of a 70-year-old female diagnosed with scleroderma (diffuse SSc) at the age of 68. She had a previous diagnosis of primary biliary cholangitis when she was 52 years old, allergic rhinitis, and was never a smoker or alcoholic drinker. Her symptoms started as pain in the shoulders and knees and swelling of bilateral hands and feet. As the disease progressed, she developed fatigue described as difficulty climbing stairs, shortness of breath, skin thickening, and weight loss of 5 kg in 1 month. Raynaud’s syndrome was also noted then. She consulted with an immunologist, and a workup was done with an antinuclear antibody and anti-RNA polymerase III antibody test, revealing positive results. Videocapillaroscopy showed a scleroderma pattern. A thoracic computed tomography (CT) scan showed accentuation of the pulmonary bronchovascular interstitium. Given the association between the SSc, especially with positivity for RNA III polymerase and neoplastic disease, other workups included a Doppler echocardiogram considered normal with elevated borderline artery systolic pressure, an esophagogastroduodenoscopy with grade A peptic esophagitis and Schatzki’s ring. Further investigations for malignancy potential were done (e.g., mammograms, breast ultrasound, thoracic-abdominal-pelvic CT scans, colonoscopy), all with negative results. She was non-steroidal started anti-inflammatory drugs and prednisolone 5 mg/day.
One month after the treatment initiation, the disease progressed with sclerodactyly and more marked skin thickening with microstomia. Treatment was shifted to mycophenolate mofetil 2.5 g/day but without symptom improvement. A positron emission tomography (PET)-CT showed negative results for distal involvement or hypermetabolism. Treatment was shifted to monthly cyclophosphamide for nine cycles. One year after, a non-specific interstitial change pattern was seen on a thoracic CT scan; her treatment was shifted to nintedanib and mycophenolate mofetil for pulmonary fibrosis.
Monitoring scans during treatment were continued showing a suspicious liver lesions. Two years after diagnosis, an abdominal magnetic resonance imaging (MRI) scan showed five nodular lesions in the right liver, the two most extensive measuring approximately 54 mm × 46 mm in segment VII/VI and 14 mm in segment VIII [Figure 1]. A biopsy confirmed carcinoma of mammary gland origin, lobular morphology (CK7+, CK20, GATA-3 +, SOX-10-, TTF-1 negative, SATB2 negative, PAX-8 negative, synaptophysin, and E-cadherin negative), estrogen receptor 90%, and human epidermal growth factor receptor 2 negative (0 score). A breast MRI showed a small lesion in the left breast (5.5 mm) below the inferior areola. Further imaging with a PET-CT scan revealed osteomedullary foci on the left shoulder and left tibia. A thoracic CT scan showed a slight worsening of the previous fibrosis findings.
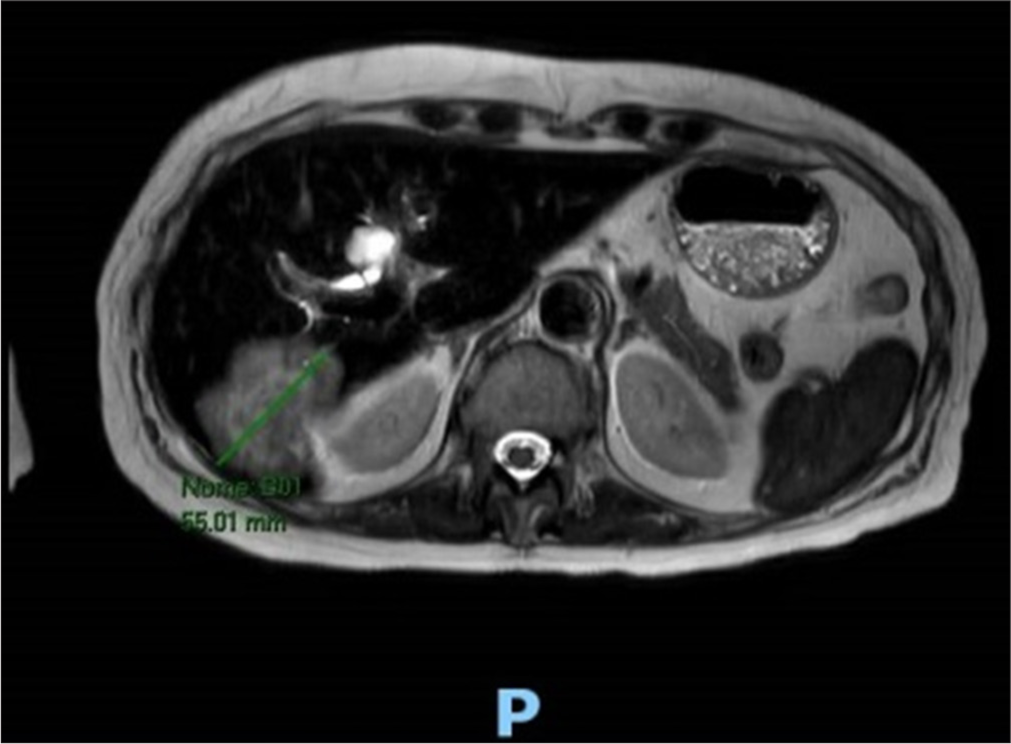
- Magnetic resonance imaging scan showing lesions in the liver before the oncologic treatment. The green arrow shows the size of the mass seen in the liver at segment VII/VI measuring approximately 55mm.
The neoplasm was staged as metastatic disease and started on 1st line ribociclib with letrozole. Six months after the treatment initiation for the breast carcinoma, there was a dimensional and numerical reduction of focal hepatic lesions. A second MRI of the upper abdomen 11 months after the oncologic initiation showed a response to targeted and hormonal therapy with partial response in some of the liver lesions. The last chest CT scan showed superimposed interstitial changes. She is currently continuing her treatment for breast cancer with regular follow-ups with the medical oncologist and immunologist.
DISCUSSION
Paraneoplastic rheumatic disorder is a clinical syndrome that may present before, during, or after the diagnosis of cancer. The model of cancer-induced autoimmunity is most compelling in SSc (scleroderma) and DM. In our case presented, we see that breast cancer, as the underlying cause, played a role in the development of auto-immunity.
Several case reports have recognized the association between SSc and malignancy. Shah et al. correlated the alteration of gene encoding RNA polymerase III (i.e., POLR3A gene locus) in tumors that trigger autoimmunity.[4] The development of polymerase autoantibodies reacts with mutation-specific T cells, triggering the development of anti-tumor immunity.[4] Mutated POLR3A gene, either through somatic mutation or loss of heterozygosity, was found in patients with antibodies to RNA polymerase III but not in cancer with other autoantibodies specificities.[5] In a matched case–control study of 50 breast cancer patients without rheumatic disease, no subject had moderate or strong autoantibody positivity, thereby confirming the specificity of these autoantibodies as a cancer biomarker only in patients with rheumatic diseases.[6]
Immunoediting explains the temporal event on cancer development and immune response. Schreiber et al. proposed the 3 stages of immunoediting: (1) Elimination in which the immune response dominates resulting in elimination of cancer, (2) Equilibrium wherein the anti-cancer immune response and cancer are balanced, and finally, (3) Es cape representing the cancer dominating the immune response and more immune checkpoints are expressed.[7] The concept of immunoediting, temporal clustering of cancer, and rheumatic diseases suggests that cancer autoantigens initiate the immune response.
There are other autoantibodies involved in the pathogenesis of scleroderma and malignancy, such as oncoproteins, tumor suppressor genes (P53), proliferation-associated antigens, and onconeural antigens.[8] However, their clinical significance is yet to be determined.
Previous epidemiologic studies discussed that patients with scleroderma are at increased risk for malignancy. In a cohort study by Derk et al., 10% of total cancer patients develop scleroderma as paraneoplastic, most prevalent in breast cancer.[9] Although, in our case, it is the underlying malignancy that triggers the autoimmunity, patients with scleroderma may also have an elevated risk of cancer due to the disease activity. Some tumors may not be apparent at the time of rheumatic disease diagnosis due to robust immune response. Other known factors such as cytotoxic therapies used to treat scleroderma, chemical exposure, and genetic predisposition. Of note, patients with cancer may develop scleroderma due to cancer treatment.
Recent reports have shown that inhibiting cyclin-dependent (CDK4/6) influences anti-tumor immune response, acting both on the cells and immune environment.[10] CDK4/6 inhibitors produce an immunomodulatory effect. Increased presentation of tumor antigens, recruitment of immune cells by senescence-associated secretory phenotype, effector T-cells activation, depletion of immunosuppresive regulatory T cells (Tregs), and enhancement of macrophage and dendritic cells antigen highlight the importance of unleashing immunity in cancer treatment.
This shed a light that our case is a paraneoplastic origin since CDK4/6 inhibitors usually upregulate immune response and should have produced “hyperimmunity,” aggravating her current SSc through T cell activations. As she responded from her cancer treatment, we theorize that the cancer cell’s antigens drove the development of SSc. Clinicians must balance the benefits of these treatments with the severe side effects, which can lead to recurrence and treatment failure, especially in metastatic settings.
CONCLUSION
There is substantial evidence of a relationship between SSc and malignancy. Autoantibodies play a vital role in the development of autoimmunity, though they are nonspecific or sensitive. They may be apparent in some rheumatologic conditions, while they are less common in others. The presence of risk factors and non-responsiveness to conventional SSc treatment signifies that an underlying malignancy can be a potential cause.
Acknowledgment
The authors would like to acknowledge the patient for her permission to publish the case, the oncology, supportive care, and imaging department of Champalimaud Foundation’s Breast Unit for their expertise in managing the case. The main author would also like to thank the European Society of Medical Oncology (ESMO) and the International Cancer Foundation (ICF) for the Clinical Unit Visit opportunity at the time of writing this paper.
Ethical approval
The research/study complies with the Helsinki Declaration of 1964.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Systemic sclerosis: A prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557-67.
- [CrossRef] [PubMed] [Google Scholar]
- Is systemic sclerosis an antigen-driven T cell disease? Arthritis Rheum. 2004;50:1721-33.
- [CrossRef] [PubMed] [Google Scholar]
- Paraneoplastic sclerodermiform syndrome-case report. Acta Reumatol Port. 2014;39:87-90.
- [Google Scholar]
- Brief report: Anti-RNPC-3 antibodies as a marker of cancer-associated scleroderma. Arthritis Rheumatol. 2017;69:1306-12.
- [CrossRef] [PubMed] [Google Scholar]
- Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science. 2014;343:152-7.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-RNA polymerase III antibodies in patients with suspected and definite systemic sclerosis: Why and how to screen. J Scleroderma Relat Disord. 2018;3:214-20.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-70.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer and autoimmunity: Autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis. 2001;60:433-41.
- [CrossRef] [PubMed] [Google Scholar]
- A cohort study of cancer incidence in systemic sclerosis. J Rheumatol. 2006;33:1113-6.
- [Google Scholar]
- Systemic sclerosis association with malignancy. Clin Rev Allergy Immunol. 2022;63:398-416.
- [CrossRef] [PubMed] [Google Scholar]







