Translate this page into:
Biomarkers in immuno-oncology: A review article

*Corresponding author: Dr. Amol Patel, Department of Medical Oncology, Malignant Disease Treatment Centre, Army Hospital, Research and Referral, New Delhi - 110 010, India. dr.amolpatel@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Patel A, Soneji D, Parikh P, Kumar M. Biomarkers in immuno-oncology: A review article. Int J Mol Immuno Oncol 2019;4(2):41-9.
Abstract
Immunotherapy is the new addition to the armamentarium against cancer. Role of immune checkpoint inhibitors is proven, but with limitations of benefit in around 20% of the patients. The cost of therapy and to some extent the toxicities are deterrent for generalized use. Biomarker is urgently needed to rationalize the use of immunotherapy. Tumor mutational burden, programmed death-ligand 1 expression, microsatellite instability, tumor-infiltrating lymphocytes, T-cell repertoire, and various other biomarkers have shown early promise in predicting response and benefit from immune checkpoint inhibitors. Majority of studies are limited by their retrospective nature, small number, and lack of prospective validation in large cohorts. Precision oncology is desired at every level including patients, doctors, and health-care system of a nation. Here, we concisely reviewed literature.
Keywords
Immune checkpoint inhibitors
Tumor mutational burden
Programmed death-ligand 1
Microsatellite instability
Overall survival
INTRODUCTION
Immunotherapy is the new addition to the armamentarium in fight against cancer. In 2018, the Nobel prize for medicine was awarded jointly to Professor James P. Allison and Professor Tasuku Honzo for their discovery of immunotherapy.[1] Worldwide, the usage of immunotherapy is increasing and it will reach to USD 201 billion by 2021.[2] Anti-programmed death (PD1) antibodies, nivolumab and pembrolizumab, and anti-programmed death-ligand 1 (PD-L1) antibody, atezolizumab, are widely used in various malignancies. The overall response rates are around 20% across all lung cancer trial of immunotherapy and overall survival gain is in the range of 2–3 months, some of the responding patients keep benefitting for a longer time.[3,4] Precision oncology is desired at every level including patients, doctors, and health-care system of a nation in consideration of cost. For rationalization of immunotherapy, biomarkers are needed to find out the patients who will benefit or who will not. Biomarkers are urgently needed for targeting the bull’s eye. Various biomarkers such as tumor mutational burden (TMB), PD-L1 expression, genetic aberration of immune-related genes, T-cell repertoire, and CD4/CD8 cells ratio are being studied. Here, we concisely reviewed literature.
WHAT IS A BIOMARKER OR CANCER BIOMARKER?
The National Institute of Health defines biomarker as “a biological molecule found in blood, other body fluids, or tissues that are a sign of a normal or abnormal process or of a condition or a disease.”[5,6] The biomarker may be aberrant translational product of a gene and may be genetic or somatic aberration for cancer specifically. Biomarker has the potential role to guide for accurate diagnosis, prognostication, prediction of response to treatment, and follow-up post- treatment. For precision oncology, a biomarker is desired specifically for prediction of response to immunotherapy. Multiple biomarkers are studied in the past decade with limitations like majority are of retrospective in nature and with limited clinical utility. Among the biomarkers in immune-oncology, TMB and PD-L1 come at priority.
TMB
As tumor proliferates, cell acquires mutations (mut) and while doing so, neoantigens are formed which may lead to response to immune checkpoint inhibitors (ICIs).[7] TMB is an emerging, novel predictive marker for immunotherapy and it is defined as number of somatic mut, short insertions, deletions, and substitution of coding base per megabase (Mb) of genome examined.[8,9] TMB is measured by whole exome sequencing (WES) and considered as the gold standard. WES is time consuming (turnaround time), carries high cost, and requires of fresh tissues limiting its use.[10] WES has not been optimized for clinical utility. Targeted next- generation sequencing (NGS) uses selected gene panel and provides as sensitive analysis as WES for predicting response to ICIs.[11] Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) (468 genes) and FoundationOne companion diagnostic (CDx) (324 genes) are two extensively studied targeted NGS methods, wherein formalin-fixed samples are used. The US Food and Drug Administration (FDA) has approved the use of FoundationOne CDx and MSK-IMPACT for clinical use.[12,13] FoundationOne CDx has been approved for use in five diseases, namely non-small cell lung cancer (NSCLC), breast cancer, colon cancer, ovarian cancer, and melanoma. In Foundation Medicine database of 104,814 cases with > 10 mut/Mb in 30 solid tumors types, average was 13.3% across all 30 solid tumor types, implying possible future use of ICIs in these tumors.[14] Various studies have highlighted the importance of TMB as predictive marker in various malignancies [Table 1]. Across all these studies, TMB has been proved as predictive biomarker but not as a prognostic marker.
| Study | Method of TMB | Trial highlights | Results |
|---|---|---|---|
| Rizvi et al.[8] | WGS Discovery cohort median mut – 302 in durable clinical benefit 148 in no durable benefit Validation cohort 244 – durable clinical benefit 125 – no durable benefit | mNSCLC Pembrolizumab Validation (n=18) and Discovery cohort (n=16) | Non-synonymous high burden ORR 63% versus 0% (P=0.03) mPFS 14.5 versus 3.7 months P=0.01, HR 0.19 (CI0.05–0.70) |
| Hellman et al.[9] CheckMate 227 Multipart study | FoundationOne CDx≥10 mut/Mb | mNSCLC Phase 3, first line Nivolumab+Ipilimumab (n=576) versus chemotherapy (n=570) | Result among patients with high TMB ORR – 45.3% versus 26.9%. 1-year PFS rate- 42.6% versus 13.2% mPFS – 7.2 versus 5.5 months Disease progression or death HR 0.58 (CI 0.41–0.81) P<0.001 |
| Carbone et al.[15] CheckMate 026 | WGS mut- 0–100: Low ≥101–242: Medium 243: High (FoundationOne correlation with WGS was done) | mNSCLC Phase 3, first line Nivolumab (n=271) versus chemotherapy (n=270) | Exploratory analysis for high TMB ORR 47% versus 28% PFS 9.7 versus 5.8 months No OS difference crossover 68% of patients received Nivolumab |
| Alexandra Synder et al.[16] | WGS Mutational load cutoff 100. Discovery cohort>100 mut (n=17) ≤100 (n=8) | Malignant melanoma Ipilimumab or tremelimumab | Mutational load was associated with clinical benefit (P=0.01) High mutational burden had improved OS (P=0.04) (*there were tumors with high mutational burden did not respond to therapy) |
| Jonathan et al.[17] | Targeted panel 315 genes n=150 | Metastatic urothelial cancers Phase 2 trial Atezolizumab | Median mutational load in responders was 12.4/Mb against 6.4/Mb in non- responders |
| Powles et al.[18] IMvigor211 trial Phase 3 | Foundation one High TMB (at or above the median) (n=274) Low TMB (below the median) (n=270) | Atezolizumab versus chemotherapy in platinum refractory urothelial carcinoma | Median TMB –9.65/Mb High TMB –median OS 11.3 months versus 8.3 months (numerically high) for atezolizumab arm. Low TMB – 8.3 versus 8.1 months |
| Hellman et al.[19] CheckMate 032 Exploratory analysis | WGS Tertiles Low – 0–< 143 mut Medium 143–247 High≥248 | SCLC Nivolumab (n=133) Nivolumab+Ipilimumab (n=78) | 1-year OS rate High mut – 35.2% Nivolumab alone versus 62.4% Nivolumab+Ipilimumab Medium 26% versus 19.6% Low 22.1 versus 23.4% |
OS: Overall survival, TMB: Tumor mutational burden, Mb: Megabase, mut: mutation, mNSCLC: Metastatic non-small cell lung cancer, CI: Confidence interval, PFS: Progression-free survival
Yarchoan et al. did the analysis for 27 tumor types of TMB and response rates. Maximum responses were seen in cutaneous squamous cell carcinoma, Merkel cell carcinoma, urothelial carcinoma, lung cancer, and colorectal carcinoma with mismatch repair deficient. Remarkably pancreatic, germ cell tumors had low TMB and low response rates. They observed statistically significant correlation with TMB and objective response rates (P < 0.001).[20]
Seminal work by Rizvi et al. established the importance of TMB and its relation to response to ICIs in patients of lung cancer with pembrolizumab.[8] In a pilot study of neoadjuvant immunotherapy for resectable lung cancer, wherein two doses of immunotherapy were given before surgery at a dose of 3 mg/kg/q2 weeks, major pathological response was seen in patients with mean mut of 311 ± 55 as compare 74 ± 60 which was statistically significant (P = 0.01).[21] Majority of the studies are pilot studies, exploratory or retrospective analysis of tumor samples of a prospective study. Other major concern of TMB is the methodology and diligent and meticulous aspects of procedures.
For TMB analysis, various parameters are important such as limit of detection, deamination artifacts, assessment of tumor cellularity, use of fresh sample, or formalin-fixed specimens[10] [Figure 1].
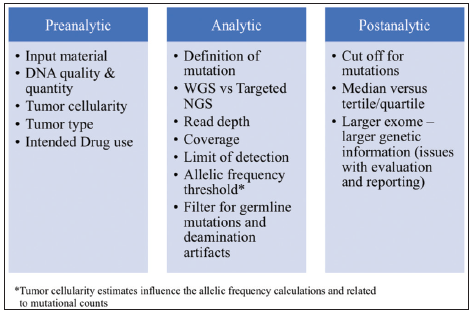
- Factors influencing the estimation of tumor mutational burden.
Various questions do arise for TMB and it is on ground usage. The cutoff values for mut do differ by techniques used and also for tumor types. What standard cutoff should be used? For FoundationOne CDx, the cutoff usually considered is 10 mut/Mb for metastatic NSCLC (mNSCLC) and MSK- IMPACT used cutoff 7.4 mut/Mb.[11]
Does this apply to all malignancies? What is the limit of genes examined? The pool of genes is getting increased in both the approved techniques. Will there be a fixed cut off? Another important aspect of germline variant? How to filter out and filter in these important findings? Finally, for evidence-based medicine, how are these laboratories are going to validate the findings in various tumor types and with various cutoffs?
Till date, there is no regulatory approval for the use of TMB guided use of ICIs. However, it holds promise for future precision immune-oncology once aforementioned gets answered by prospective validation studies.
PD-L1 EXPRESSION
PD1 (CD279) is a surface receptor predominantly on normal T cells which was initially thought to regulate cell death. However, now, it is well recognized for an important role in immune checkpoint inhibition. This cell surface receptor is part of superfamily of immunoglobulins. Similarly, its ligand PD-L1 (CD274) is expressed by various immune regulatory cells such as antigen-presenting cells, macrophages, and dendritic cell. This immune mechanism is tightly regulated in normal homeostasis. Tumor cells may express PD-L1 and these ligands downregulate T-cell activation. To prevent this immune evasion of tumor cells, monoclonal antibodies against PD1 and PD-L1 are developed and received approval for clinical use.
To surprise, the utility of PD-L1 as a biomarker is challenged as tumor cells expressing < 1% also showed response to ICIs. As per meta-analysis of eight recent trials of ICIs (n = 4174), tumor expressing < 1% of PD-L1 had overall survival better than chemotherapy with HR of 0.80 (confidence interval [CI]: 0.71–0.90) as it was for ≥1% PD-L1 expression, HR is 0.66 (CI: 0.59–0.74).[22]
The use of PD-L1 expression as a biomarker is prospectively studied in pembrolizumab trials, the inclusion criteria for the first-line and second-line lung trial, the expression of PD- L1 should be more than ≥ 50% and ≥ 1%, respectively.[23,24] However, across all ICIs trial in lung cancer, the pertinent finding emerged is the benefit of ICIs irrespective of PD-L1 expression [Table 2].
| PD-L1 expression (n) | OS (months) | HR | CI | Results | |
|---|---|---|---|---|---|
| KEYNOTE 042[25] mNSCLC Pembrolizumab versus platinum doublet chemotherapy Treatment naïve At least 1% PD-L1 |
≥ 50 (599) ≥ 20 (818) ≥ 1 (1274) |
20 versus 12 18 versus 13 17 versus 12 |
0.69 0.77 0.81 |
0.56–0.85 0.64–0.92 0.71–0.93 |
PD-L1 expression 1–49%, OS 13.4 versus 12.1 months (HR-0.91) |
| KEYNOTE 407 (n=559)[26] Squamous mNSCLC Doublet+Pembrolizumab versus doublet chemotherapy Treatment naïve PD-L1 not selected |
≥ 50 (146) 1–49 <1 |
NR versus NR 14 versus 11.6 15.9 versus 10.2 |
0.64 0.57 0.61 |
0.37–1.10 0.36–0.90 0.38–0.98 |
No statistical significance in OS limited by small number of patients |
| KEYNOTE 189 (n=616)[27] Non–squamous PD-L1 unselected Pemetrexed+platinum With or without pembrolizumab |
≥ 50 1–49 <1 |
*73 versus 48.1 71.5 versus 50.9 61.7 versus 52.2 |
0.42 0.55 0.59 |
0.26–0.68 0.34–0.90 0.38–0.92 |
No interaction between these subgroups |
| KEYNOTE 024 (n=305)[28] PD-L1 ≥ 50% mNSCLC, pembrolizumab versus platinum doublet |
≥ 50 | 30 versus 14.2 | 0.63 | 0.47–0.86 | Preliminary results at a follow of 25 months |
| KEYNOTE 10[24] Second line PD-L1 ≥1, Pembrolizumab (2 and 10 mg/kg dose) versus chemotherapy |
1–49 ≥ 50 |
14.9 (#17.3) versus 8.2 NA |
0.76 0.53 |
0.60–0.96 0.40–0.70 |
Significant benefit for non-squamous histology |
| CheckMate 026[15] Treatment naïve mNSCLC, PD-L1>1% 78% >5% Nivolumab versus chemotherapy |
≥ 5% ≥ 50% |
14.4 versus 13.2 15.9 versus 13.9 |
1.02 0.90 |
0.80–1.30 0.63–1.29 |
PFS was numerically higher in chemotherapy group by 1.7 months |
| CheckMate 227[9] Treatment naïve Multipart study Nivolumab+Ipilimumab versus chemotherapy |
≥ 1 <11 |
NA NA |
0.62 0.48 |
0.44–0.88 0.27–0.85 |
Data analyzed for PFS. PFS was better in combination arm irrespective of PD-L1 expression |
| CheckMate 227[29] Treatment naïve multipart study, n=363 <1% PD-L1, Nivolumab+Chemotherapy versus chemotherapy |
< 1 | NA | 0.74 | 0.58–0.94 | 5.6 versus 4.7 months PFS in combination arm |
| CheckMate 017[30] Second line Squamous Nivolumab versus docetaxel |
< 1 ≥ 1 ≥ 10 |
- - - |
0.58 0.69 0.50 |
0.37–0.92 0.45–1.05 0.28–0.89 |
PD-L1 neither prognostic or predictive marker |
| CheckMate 017 plus 057[31] combined analysis Second line Squamous and non-squamous Nivolumab versus chemotherapy | <1 (162) ≥ 1 (186) ≥ 5 (137) ≥10 (122) ≥ 50 (83) |
9.6 versus 7.8 13.4 versus 8.5 17.2 versus 7.7 17.5 versus 7.7 20.6 versus 8 |
0.78 0.67 0.51 0.47 0.42 |
0.61–0.99 0.53–0.85 0.38–0.670.35–0.630.28–0.63 |
2-year OS rate was better in nivolumab arm. In non-squamous histology, higher PD-L1 was associated with greater OS benefit. In squamous, benefit was irrespective of PD-L1 expression |
| OAK trial[32] Atezolizumab versus chemotherapy Second-/third-line mNSCLC |
TC0 and IC0 TC or IC 1/2/3 TC or IC 2/3 TC or IC 3 |
12.6 versus 8.9 15.7 versus 10.3 16.3 versus 10.8 20.5 versus 8.9 |
0.75 0.74 0.67 0.41 |
0.41–0.640.49–0.900.58–0.93 0.59–0.96 |
OS benefit is irrespective of PD-L1expression |
NA: not available, *OS rate in %. #10 mg/kg. OS: Overall survival, PD-L1: Programmed death-ligand 1, ICIs: Immune checkpoint inhibitors, mNSCLC: Metastatic non-small cell lung cancer, PFS: Progression-free survival
In CheckMate 017 trial, P value for interaction at cutoff level of 1, 5, and 10% for overall survival was 0.55, 0.47, and 0.40, respectively. Similar non-significant P values were present for progression-free survival (PFS) and objective response rates.[33] With the advent of immunotherapy in other cancer such as renal cell carcinoma (RCC), urinary bladder carcinomas, hepatocellular carcinomas, and malignant melanomas, similar finding of PD-L1 expression dissociation from the outcome variables is observed. In advanced treatment naïve RCC, data of Keynote 427 were presented ASCO 2018. Pembrolizumab as single agent has shown better response in patients of PD-L1 expression of ≥1%.[31] The combination of nivolumab and ipilimumab has shown better overall response, OS rates in intermediate and high- risk advanced RCC with PD-L1 expression ≥1% [Table 3].
| Trial | PD-L1 (%) expression | OS | HR | CI | Results |
|---|---|---|---|---|---|
| CheckMate 214[34] n=1096 Treatment naïve Nivolumab+Ipilimumab versus sunitinib |
<1 ≥1 |
74% versus 64% (at 18 months) 81% versus 53% |
0.73 0.45 |
0.56–0.96 0.29–0.71 |
Median OS – not reached in intermediate and high-risk group. Sunitinib was better in favorable risk group. PD-L1 expression was low in favorable risk (11% versus 26%) |
| CheckMate 215[35] Advanced RCC Second line Nivolumab versus everolimus | <1 ≥1 <5 ≥5 |
27.4 versus 21.2 21.8 versus 18.8 24.6 versus 20 21.9 versus 18.1 |
0.77 0.79 NA NA |
17.7–26.2 0.53–1.17 - - |
At cutoff of 5% PD-L1 expression, benefit is irrespective of PD-L1 |
| CheckMate 066[36] Advanced melanoma n=416 Treatment naïve Nivolumab versus dacarbazine | < 5 ≥5 |
NR versus 10.22 NR versus 12.39 |
0.48 0.30 |
0.32–0.71 0.15–0.60 |
PD-L1 positivity was defined for≥5% |
| CheckMate 141 Recurrent H and N[37] cancers n=361 Nivolumab versus standard of care | <1 ≥1 |
8.7 versus 4.6 months 5.7 versus 5.8 |
0.89 0.55 |
0.54–1.45 0.36–0.83 |
Benefit was irrespective of PD-L1 expression, magnitude increased with≥1% PD-L1 expression. Similar finding at PD-L1 cutoff 5% |
OS: Overall survival, PD-L1: Programmed death-ligand 1
In CheckMate 215 trial of nivolumab versus everolimus in previously treated advanced RCC, the OS benefit in nivolumab arm was irrespective of PD-L1 expression at cutoff level of 1%.[35] In a Phase 1/2 trial of nivolumab in HCC, the objective response rate was present in 26% of cases of PD-L1 ≥1% and it was 19% in < 1%.[38] In advanced melanoma treated with two doses regimen of pembrolizumab versus ipilimumab, PFS benefit was seen irrespective of PD-L1 expression and for OS, HR was 0.91 (2 weekly) and 1.02 (3 weekly) as compared with ipilimumab.[39] In CheckMate 066 trial of nivolumab in melanoma where it was compared with dacarbazine, OS benefit was irrespective of PD-L1 expression (PD-L1 positivity definition was taken for ≥5%).[36] Across all these data, though the benefit of ICIs was irrespective of PD-L1 expression, the magnitude of benefit increases with PD-L1 expression levels. Similar findings were present for advanced urothelial cancers treated with ICIs.[40] For recurrent head and neck squamous cell cancers, the OS was better in the group of composite positivity of PD-L1 ≥1% and p16 positivity.[37]
Does the concept of PD-L1 inhibitors is challenged due to no relationship with outcome? There are various explanations put forward. Tumor is heterogeneous in PD- L1 or PD-1 receptor expression. The discordance between tumor biopsy and excised specimen is known for PD-L1 expression. Majority of trials have used tissue samples retrospectively which leads to issues of tissue preservation. The tumor evolution and treatment are another speculated reason for such discrepancies. The methods used for staining (Ventana or Dako platform) and various antibodies used in different trial are another reason for heterogeneity in trial results. The cutoff levels for PD-L1 positivity differ in various trials and interobserver variability may lead to such findings.[41,42] Recent publication in cell by Lieping Chen and Mellman on fibrinogen-like protein 1 highlighted other important pathways of immune evasion and anti-PD-1 therapies.[43]
Concisely, the value of PD-L1 expression as a biomarker is limited as PD-L1 negative tumors are also benefitting in terms of objective response, PFS or OS, with one obvious caveat the magnitude of benefit rises with increasing PD-L1 expression.
TMB and PD-L1 expression – There is no direct correlation between PD-L1 expression and TMB.[9] The composite use of TMB and PD-L1 expression together predicted 50% clinical benefit rate of 50% in patients of mNSCLC.[11] This finding can be studied in future prospective trials.
MICROSATELLITE INSTABILITY (MSI)
Lynch syndrome has been known to oncology for >100 years for familial inheritance. As fragmented PCR is the routinely used test to detect MSI status, there is >90% concordance with NGS or immunohistochemistry techniques. MSI-H tumors are deficient in mismatch repair genes expression and this leads to accumulation of mutations and genomic instability. These tumors show a characteristic feature of lymphocyte infiltration in tumors that imply immunological dysregulation. Gastrointestinal and genitourinary malignancies have more MSI-H frequency as compare to others. Indeed, apart from germline mut in these DNA repair genes, somatic mut are also present in various tumor types and have been subjected to research in immunotherapy trials.
The role of MSI has been established as a predictive marker for response to ICIs in 15 different tumors types. Pembrolizumab has been approved for these tumors by FDA.[44] Approval was based on combined analysis of five Keynote trials (016, 164, 028, 012, and 158) of 149 patients of chemorefractory solid tumors in which 7.4% had complete response and 32.2% had partial response. The median duration of response was not reached (range: 1.6–22.7 months) and 78% had durable response of 6 months or more. The response in non-colorectal cancer was also promising in refractory setting. Nivolumab also showed promising responses in MSI-H colorectal cancers and received FDA approval.
The overlap of TMB, PD-L1, and MSI has been studied by Vanderwalde et al. in 2189 matched cases.[45] Only 0.6% of cases had all three markers positive and it differs among tumor types.
The peculiarity of the association of MSI-H and TMB is that majority of MSI-H tumors are associated with high TMB (97%). However, reverse corollary is not true. Only 16% of high TMB are MSI-H. This finding from FoundationOne dataset of 100,000 samples has highlighted the importance of both these parameters. In stomach, duodenum, and small intestinal adenocarcinoma, MSI-H and high TMB are mutually inclusive. However, for melanoma, squamous cell carcinoma, and lung cancers, high TMB is common, but MSI-H is uncommon among these tumors.[46]
The role of MSI is limited as biomarker due to rarity of tumor enrichment with MSI, studies are again having limitations as most of them are Phase 2 designs and represented by small number of cases in clinical trials.
TUMOR-INFILTRATING LYMPHOCYTES (TILS)
Tumor microenvironment is a subject of intense research. As only a minority of patients benefit from immunotherapy, tumor specimens have been categorized into three types of immunological profiles.[47] The tumor specimens are being studied before the start of immunotherapy. Classically, first profile is immune rich or inflamed type. Tumor stroma is enriched with CD8+ and CD4+ type of lymphocytes. Myelocytes and monocytic cells are also present. These cells are present in close approximation of tumor cell. The second immune profile is immune enriched, but the immune cells are not in close approximation of tumor cell, they are limited to surrounding stroma. The third type is immune desert, as the word suggests, it is immune cell deprived. Among all these types, it is logical and scientifically proven that the immune desert profile does not respond to ICIs.[48] The tumor immunological profile differs at primary and metastatic sites in melanoma and mNSCLC.
Tumeh et al. studied the role of CD8+ lymphocytes at tumor margin, PD-L1 expression, and response to pembrolizumab in advanced melanoma patients.[49] The CD8+ cell density at tumor margin was higher at baseline and increased in responding patients as compare to patients with lesser density of CD8+ cells. Triple-negative breast cancer (TNBC) tumors are also associated with increased lymphocytes infiltration. The PD-L1 expressing immune (macrophage, lymphocytes, neutrophils, and dendritic) cells with > 1% of cell tumors have responded better to atezolizumab as compare to ≥1%.[50] The latest article in NEJM of pembrolizumab and nab-paclitaxel in TNBC showed promising use of ICIs. The role of TILs has already been proven as prognostic in TNBC in large RCTs.[51,52] Stromal TILs have predicted response to pembrolizumab in TNBC.[53] The T-cell-inflamed gene expression profile has been studied in KEYNOTE 028, Phase 1b trial, wherein 20 solid tumors were treated with pembrolizumab and found a positive correlation with tumor response.[54]
TIL has promising prospect in predicting response to ICIs. The studies cited are either Phase 1 or retrospective in nature. To determine the role of TILs as biomarker, further prospective studies are required. As TMB and PD-L1 expression are synergistic as biomarker, the composite use of TILs and PD- L1 also needs to be studied in future.
TRANSCRIPTOMICS, T-CELL RECEPTOR CLONALITY, AND ANEUPLOIDY
Transcriptomics is the study of RNA transcripts produced by genome for a specific physiological or developmental stage and it is analyzed by microarray.[55] Hugo et al. could prove that specific transcriptomic features are associated with inherent resistance to ICIs.[56] RNA sequencing is the reference technique for analyzing the nature of tumor cells, its milieu including the infiltrating inflammatory cells, stromal cells, and stem cells. The transcriptomic nature of these cells may provide the understanding of resistance or sensitivity to ICIs in future. As the evolutionary genetics is evolving and microarray techniques have provided insight into the development of early melanoma,[57] transcriptomics will be useful tool to understand various underlying mechanisms of disease progression and immunological derangements. T-cell is the prime mediator for immunological responses.[7] T-cell receptor clonality by NGS for specific T-cell receptor V-beta CDR3 region has been studied in melanoma patients[58] and it has been shown that T-cell repertoire predicts response to ICIs in pancreatic cancer.[59] Aneuploidy is somatic copy number alternations and high aneuploidy has been associated with poor response to immunotherapy in melanoma patients in a retrospective study.[60] Aneuploidy predicts two distinct types of cancer hallmark, cell proliferation, and immune evasion and holds promise for biomarker for immunotherapy in future.
CONCLUSIONS
Regional site and disease-specific prognostic marker may be combined for biomarker development as – lung cancer – TMB and PD-L1, or head and neck cancer, PD-L1 with P16. TILs are an emerging biomarker, especially in TNBC. MSI is promising biomarker but limited by its low prevalence and confined to select tumor types, wherever it is high, ICIs have shown promising responses. TMB, PD-L1, and MSI are not overlapping and their use should be individualized for each clinical circumstance. The association of TMB and MSI is site specific. TMB high and MSI-H adenocarcinomas of stomach, duodenum, and small intestine are mutually inclusive. Transcriptomics and TILs are simple bullets in new gun with promising future. Future prospective studies are needed for correlation as a biomarker in peripheral blood and tumor T-cell receptor repertoire. Till date, no perfect biomarker has emerged from bench to bedside use for predicting response to ICIs. There is an urgent for prospective studies for validating these biomarkers and future novel markers are awaited. Until then, rational use of available biomarkers is justified.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.






