Translate this page into:
Advanced lung cancer and molecular testing in the middle of coronavirus disease 2019 second wave in the South Asian association for regional cooperation region

*Corresponding author: Purvish Parikh, Department of Medical Oncology, MOC, Mumbai, Maharashtra, India. purvish1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Parikh P, Kapoor A, Joshi K, Bansal I, Krishna V, Sahoo TP, et al. Advanced lung cancer and molecular testing in the middle of coronavirus disease 2019 second wave in the South Asian association for regional cooperation region. Int J Mol Immuno Oncol 2021;6(2):61-5.
Abstract
Objectives:
We present the survey data on molecular testing and its implications among lung cancer specialists in the South Asian Association for Regional Cooperation (SAARC) region.
Material and Methods:
An online survey Google form was developed that could be completed in <5 min. This was circulated to oncologists who had been actively participated in our academic activities and this data were collected over an 11 day period in November 2020.
Results:
We had 354 responders from 8 SAARC countries, 58.9% (209/354) being medical oncologists, and 30.1% (107/354) radiation oncologists. Nearly 50% of responders were seeing patients in the second wave of coronavirus disease 2019 and 89% had seen reduction in their lung cancer patients during first wave. Biomarker testing in advanced lung cancer using next-generation sequencing as the preferred method was more among medical oncologists (76%) as compared to all the other specialists (24%); (Pearson Chi-square = 0.006). The practice of waiting for the molecular testing report before starting first line treatment was 55.4% in corporate/private hospitals as compared to 27% in the government/charity hospital sectors (Pearson Chi-square = 0.015). A total of 89.6% responders believed that targeted therapy has resulted in improved overall survival in advanced lung cancer.
Conclusion:
Educational activities need to be strengthened so that lung cancer specialists follow comprehensive NGS testing in appropriate settings, as shown by medical oncologists
Keywords
Adenocarcinoma
Non-small cell
Pandemic
Targeted therapy
Driver mutations
INTRODUCTION
It is now well-established that molecular testing in lung cancer patients is vital to selecting the right management and can help improve overall survival as well as quality of life.[1] However, real world experience of such testing, and its impact on patient outcome is not well documented.[2,3] We, therefore, decided to conduct a survey among lung cancer specialists in the South Asian Association for Regional Cooperation (SAARC) region to document the current status.[4] Of these, 58.9% (209/354) were medical oncologists and 30.1% (107/354) were radiation oncologists.
MATERIAL AND METHODS
We devised a brief online multiple choice questionnaire for survey.[5] The questions were deliberately kept simple so that responders could complete the survey in <5 min. For the poll, a unique online Google form was developed for automatic online collection of responses (with time stamp; downloadable as a linked Google spreadsheet; Google, Mountain View, CA).[5] This online survey poll was designed for oncologists from the SAARC region to document key aspects of their real world practice in management of lung cancer patients. The survey links were shared through WhatsApp or email with oncologists who had previously registered for and participated in our online CME programs. Responses were collected between November 12, 2020, and November 22, 2020 (over a period of 11 days; at a time when only half the participants were facing the second wave of coronavirus disease 2019 [COVID-19] pandemic). The results were evaluated for completeness of response (all questions were compulsory) and duplicate entries were removed, (based on the emails provided by participants). The remaining unique answers were tabulated and analyzed. Statistical correlation analysis was performed using Pearson Chi-square test.
RESULTS
A total of 358 unique responses were received from oncologists. This included 298 from India, 56 from other SAARC Countries (Afghanistan, Bangladesh, Bhutan, Myanmar, Nepal, Pakistan and Sri Lanka) as shown in Figure 1. Four responses (one each) from Oman, UK, USA, and Australia are excluded from this analysis because they did not belong to SAARC region. Thus, our data represent 354 oncologists. Of these, 58.9% (209/354) were medical oncologists, 30.1% (107/354) were radiation oncologists, and the remaining 11% (38/354) included surgical oncologists (6.5%), pulmonologists (3.1%; involved in lung cancer management), and other oncologists (not specified).
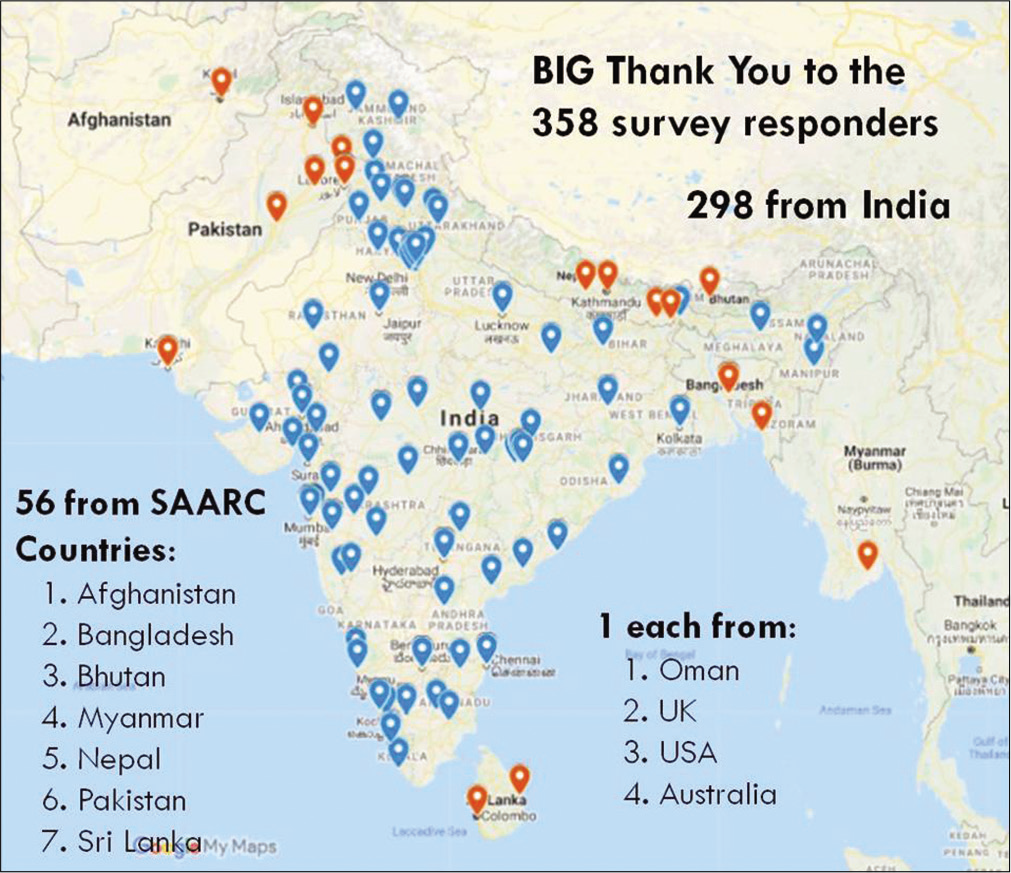
- Geographical location of participants in the online survey (Blue represents replies from India and orange indicates replies from other South Asian Association for Regional Cooperation Countries).
The majority of these specialists have been actively managing lung cancer patients for more than 10 years (43.2%; 153/354). Those practicing for 5–10 years formed the second largest group (25.4%; 90/354) and were only slightly more than those practicing since 2–5 years (20.3%; 72/354). The remaining 11% (39/354) were managing lung cancer since <2 years.
The practitioners included non-teaching setting in 50.28% (178/354) and postgraduate teaching (degree course) in 49.72% (176/354) [Figure 2]. Overall government/charitable setting were seen in 38.14% (135/354), corporate hospitals were seen in 53.1% (188/354), and the remaining 8.75% (31/354) were in solo or group practice.
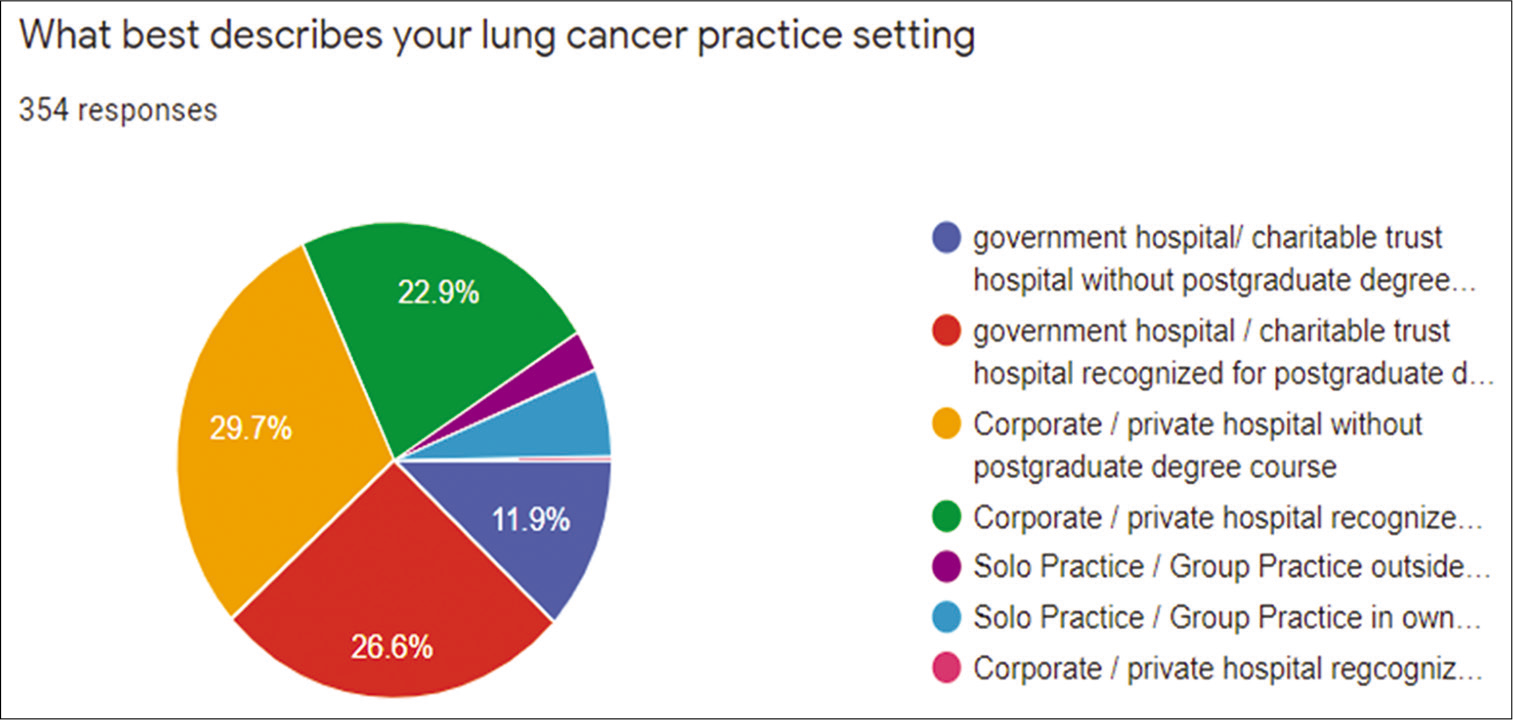
- Lung cancer practice setting in the 354 responders from South Asian Association for Regional Cooperation Countries.
While 52% (184/354) stated that they were facing resurgence of COVID-19 in the second wave, the first wave had impacted the practice of more than 89% (315/354), with 38.98% (138/354) having 25–50% reduction in the lung cancer patients; 26.8% had reduction of <25%; 16.9% had reduction of 51–75%; and 7% had reduction by more than 75%. The overall difference in the practice change did not reach level of statistical significance (Pearson Chi-square = 0.146).
While deciding first-line treatment in advanced lung cancer, the preferred method of molecular testing was simultaneous reflex testing for epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase-4 (ALK-4), ROS-1, and others in 51.7% (183/354) of responders. The second most common method was sequential testing for EGFR first and then the others (such as ALK and ROS), preferred by 25.14% (89/354) of responders. Comprehensive testing by next-generation sequencing (NGS) to look for all possible druggable mutations was done by 14.12% (50/354) of participants. EGFR testing alone was done by 26/354 (7.34%) and no molecular testing was the answer given by 7/354 (1.98%). Among those who selected the method of comprehensive NGS testing as the preferred method, the majority were medical oncologists (76%) as compared to all the other specialists (24%); Pearson Chi-square = 0.006).
After sending the sample for molecular testing, only a quarter of the responders (24.6%) actually waited for the report before commencing their patient’s treatment. The majority (71.7%) started the treatment while waiting for the report, of which, 55.6% would switch to appropriate targeted therapy if the report showed actionable mutation. The remaining 16.1% would not change treatment if the patient was having clinical benefit from the empirical treatment started. We also noted that 4% did not do molecular testing in the first-line management of advanced lung cancer. Analyzing the pattern among the specialists who waited for the molecular testing report before commencing first-line treatment, we found that 55.4% were working in corporate/private hospitals as compared to 27% belonging to the government sector (Pearson Chi-square = 0.015). This is shown in Figure 3.
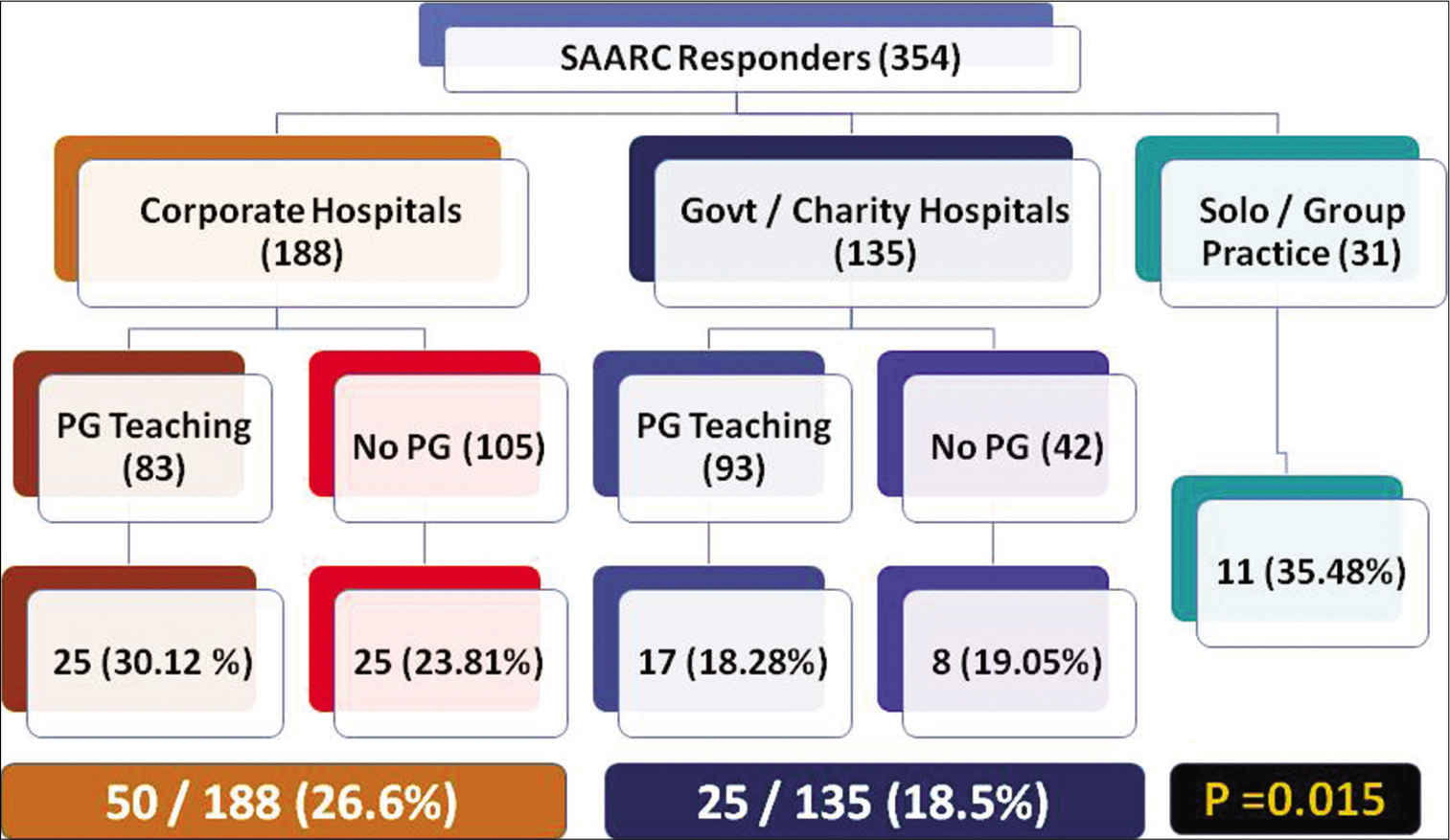
- Decision about first-line therapy in advanced lung cancer patients and its correlation with practice setting.
In response to the question regarding impact of molecular testing directed therapy on the overall survival of advanced lung cancer, the overwhelming majority (73.8%) said it increased by 1–50% (43.7% choosing increase of 26–50%; and 30.1% selecting increase up to 25%). Another 15.8% mentioned increased OS of 51–100%. At the extreme of the spectrum, 6.5% believed that OS has improved by >100% where as 3.9% were of the opinion that molecular testing did not improve OS at all. Eighty percent of those who had ordered NGS comprehensive testing had belief that molecular testing had led to improved survival by >25% (Pearson Chi-square = 0.01).
DISCUSSION
Lung cancer is a very important component of the oncology burden, not only globally but also in the SAARC region. For instance, as per Indian Council for Medical Research data (2020), India sees 14,00,000 new cancer cases of which lung cancer will form 1,68,000 (12% of all cancers).[6] Non-small cell lung cancer will be 142,000 (85%) of all the lung cancers and 68,544 (48%) of them will present in the metastatic setting. These will require systemic cancer directed therapy as the primary modality of treatment and 36% (24,676) are expected to have targetable driver mutations – which can only be identified by molecular testing.
SAARC countries face several limitations, mainly based on economic restrictions as well as pharmacogenomic differences.[1] Our survey was designed to document the real world status of advanced lung cancer management in our region. Since it includes 298 from India as well as 56 from seven other SAARC countries, it is a fair representation. The majority of responders were medical oncologists (58.9%) and radiation oncologists (30.1%), who primarily manage advanced lung cancer in South Asia.
Our survey was conducted at a time when half the participants were facing the second wave of COVID-19. Majority (89%) of them had a significant reduction in their lung cancer practice during the first wave. However, the overall difference in the practice change did not reach level of statistical significance (Pearson Chi-square = 0.146).
It is well-established that lung cancer patients require biomarker testing driven personalized medicine. However, in the real world it’s application faces limitations such as availability of testing facilities, turnaround time, quality assurance, and high cost.[7] Hence, it is not surprising that molecular testing in SAARC countries is highly variable. The most common method still remained reflex testing for EGFR, ALK-4, and ROS-1 mutations for 51.7% (183/354) patients. While international guidelines consider comprehensive NGS testing as standard of care, it is used by only 14.12% of participants – the majority of them being medical oncologists (76%) and this was statistically significant (Pearson Chi-square = 0.006).[8]
The purpose of molecular testing is to guide patient treatment. Surprisingly only 24.6% of responders waited for the report before starting treatment. There was significant difference in the approach between those working in the corporate setting versus those in government/charitable hospitals – with the former complying with this recommendation (in 55.4%; Pearson Chi-square = 0.015) [Figure 3]. It is possible that conditions of some patients warranted immediate treatment. This could be related to advanced disease at presentation requiring immediate treatment. Hence, it was good to note that 55.6% would switch to the targeted therapy indicated by the biomarker test report.
The ultimate objective of managing lung cancer is to improve their chance of cure, overall survival and/or quality of life. Today, there is ample evidence that targeted therapy directed by molecular testing improves overall survival. It is logical to expect that those who order molecular testing and use its report to decide appropriate testing would be those who believe that such an approach improves survival. This hypothesis was proved to be correct, since 80% of those who were in favor of NGS comprehensive testing, also believed that it led to improved survival by >25% (Pearson Chi-square = 0.01).
Our survey data documents the diversity in the perception about value of molecular testing and its appropriate utilization to direct treatment of advanced lung cancer in the SAARC region. It identifies areas needing focus for revised educational strategies as well as improvement in infrastructure and reduction in cost to increase availability of testing. Attention to these unmet needs will facilitate optimal management of our patients with advanced lung cancer.
CONCLUSION
Molecular testing for lung cancer is highly variable amongst SAARC Countries. Most oncologists perform reflex testing for EGFR, ALK, and ROS mutations. As accepted standard of care, NGS testing is underutilized but most common amongst medical oncologists. Appropriate biomarker testing is vital to select the most appropriate treatment and optimize outcome in our patients with lung cancer. Educational strategies need strengthening to achieve this goal amongst South Asian nations.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
Nil.
References
- Personalized medicine: Lung cancer leads the way. Indian J Cancer. 2013;50:77-9.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular Predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034-42.
- [CrossRef] [PubMed] [Google Scholar]
- Lung cancer in India: Current status and promising strategies. South Asian J Cancer. 2016;5:93-5.
- [CrossRef] [PubMed] [Google Scholar]
- Afro Middle East Asian symposium on cancer cooperation. South Asian J Cancer. 2014;3:128-31.
- [CrossRef] [PubMed] [Google Scholar]
- Practical consensus recommendations-current role of immune response evaluation criteria in solid tumors criteria in the management of cancer patients receiving immunotherapy. Int J Mol Immunol Oncol. 2019;4:21-3.
- [CrossRef] [Google Scholar]
- Available from: https://www.ncdirindia.org/all_reports/report_2020/default.aspx [Last accessed on 2020 Dec 12]
- Efficacy and safety of short course adjuvant trastuzumab combination chemotherapy in breast cancer. South Asian J Cancer. 2017;6:47-50.
- [CrossRef] [PubMed] [Google Scholar]
- Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: A KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol. 2020;31:191-201.
- [CrossRef] [PubMed] [Google Scholar]







