Translate this page into:
Immune checkpoint inhibitor-induced acute fulminant myocarditis: A case report and review of literature

*Corresponding author: Dr. Shirish Alurkar, MD, Department of Medical Oncology, HCG Aastha Cancer Centre, Ahmedabad, India. ssalurkar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vyas M, Patel V, Shah M, Alurkar S. Immune checkpoint inhibitor-induced acute fulminant myocarditis: A case report and review of literature. Int J Mol Immuno Oncol. 2025;10:45-9. doi: 10.25259/IJMIO_33_2024
Abstract
Immune checkpoint inhibitor (ICI)-induced myocarditis is a rare but fatal cardiotoxicity of ICIs. It is an early toxicity of ICIs, occurring within the first 30–40 days of starting the therapy. The exact mechanism is unknown but is postulated to be due to the activation of the CD4+ and CD8+ T-lymphocytes in the myocytes. Patients may have subtle changes in the electrocardiogram (ECG) and echocardiography during therapy with some patients presenting with acute left ventricular failure after the first or second dose of the ICI. We present a case of hepatocellular carcinoma developing severe fulminant ICI-induced myocarditis after 2 doses of the ICI. A high index of suspicion is needed to detect the toxicity early on and the treatment protocol should include an ECG and troponin levels at each cycle in the initial phases of treatment. The mainstay of treatment is high doses of steroids and other cardiac support measures.
Keywords
Cardio-oncology
High-dose steroids
Immune checkpoint-induced myocarditis
Myocarditis
INTRODUCTION
Immune checkpoint inhibitors (ICI) are gradually becoming a mainstay modality of treatment in many cancers. Apart from their use in advanced or recurrent cancers, they are increasingly being used in adjuvant and neoadjuvant settings. Single-agent ICIs or in combination with chemotherapy or radiotherapy is also being established as a standard of care. They have improved disease-free survival and overall survival in many cancers. Usually, the incidence of immune-related adverse effects (irAE) is less (0.5–10%) and comprises hypothyroidism, gastrointestinal, hepatic, pulmonary, and dermatological toxicities.
Cardiac toxicities due to ICI are rare and comprise arrhythmias, bundle branch blocks, complete heart block, pericarditis, and even myocarditis. Acute ICI-induced myocarditis is a rare irAE (0.09–1.5%) which can be fatal in half the cases.[1] It can be detected early on during the treatment and the risk of fatal myocarditis can be mitigated. A high index of suspicion is needed to detect early cardiotoxicity.
We present here a case of hepatocellular carcinoma on ICI and monoclonal antibody against vascular endothelial growth factor (anti-VEGF) developing a fatal fulminant acute myocarditis. We also review the literature with respect to pathophysiology, clinical features, incidence, and how to detect subtle cardiac toxicity of ICIs before the patient becomes symptomatic.
CASE REPORT
A 77-year-old gentleman was diagnosed in August 2024 to have a moderately differentiated hepatocellular carcinoma of the liver. The alpha-fetoprotein level was >4500 IU/mL with normal carbohydrate antigen - 19.9 and carcinoembryonic antigen levels. A triple-phase computed tomography scan showed a 9.7 × 6 × 8.3 cm lesion in the left lobe of the liver with contrast enhancement and early washout in the venous phase. There were also changes of cirrhosis in the liver with a Child-Pugh A score. A biopsy of the liver with immunohistochemistry confirmed a hepatocellular carcinoma. The viral markers (human immunodeficiency virus, hepatitis B surface antigen, and hepatitis C virus) were negative. Esophagoscopy did not reveal any varices. A staging positron emission tomography scan on September 02, 2024, showed a high-grade FDG-avid lesion (125 × 72 × 95 mm, SUV max 22.8) in segment II of the left lobe of the liver with multiple FDG-avid periportal, para-aortic, and inter-aortocaval nodes [Figures 1 and 2]. His electrocardiogram (ECG) was normal and a 2D echocardiography with an ejection fraction (EF) of 60% with a global longitudinal strain of −20%.
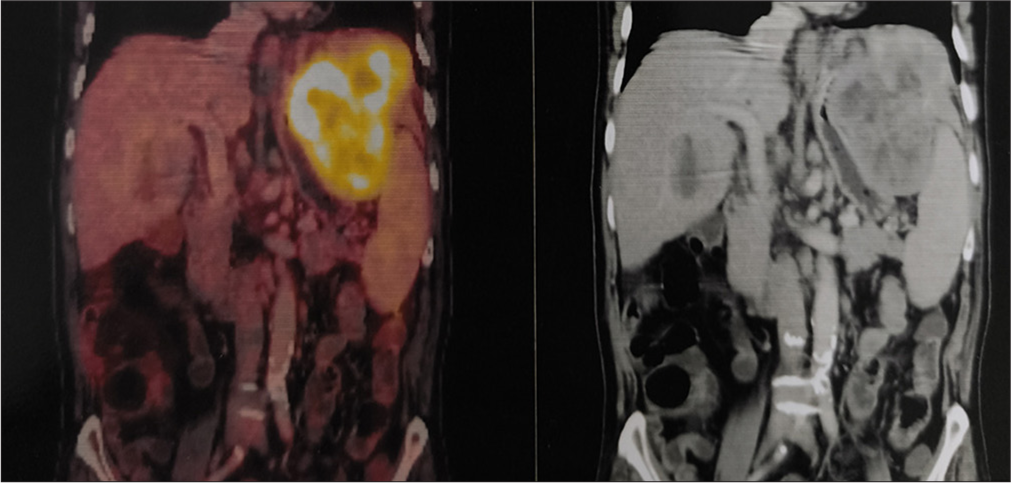
- Coronal view of an fluorodeoxy glucose (FDG)-positron emission tomography scan showing a large mass in the left lobe of liver.
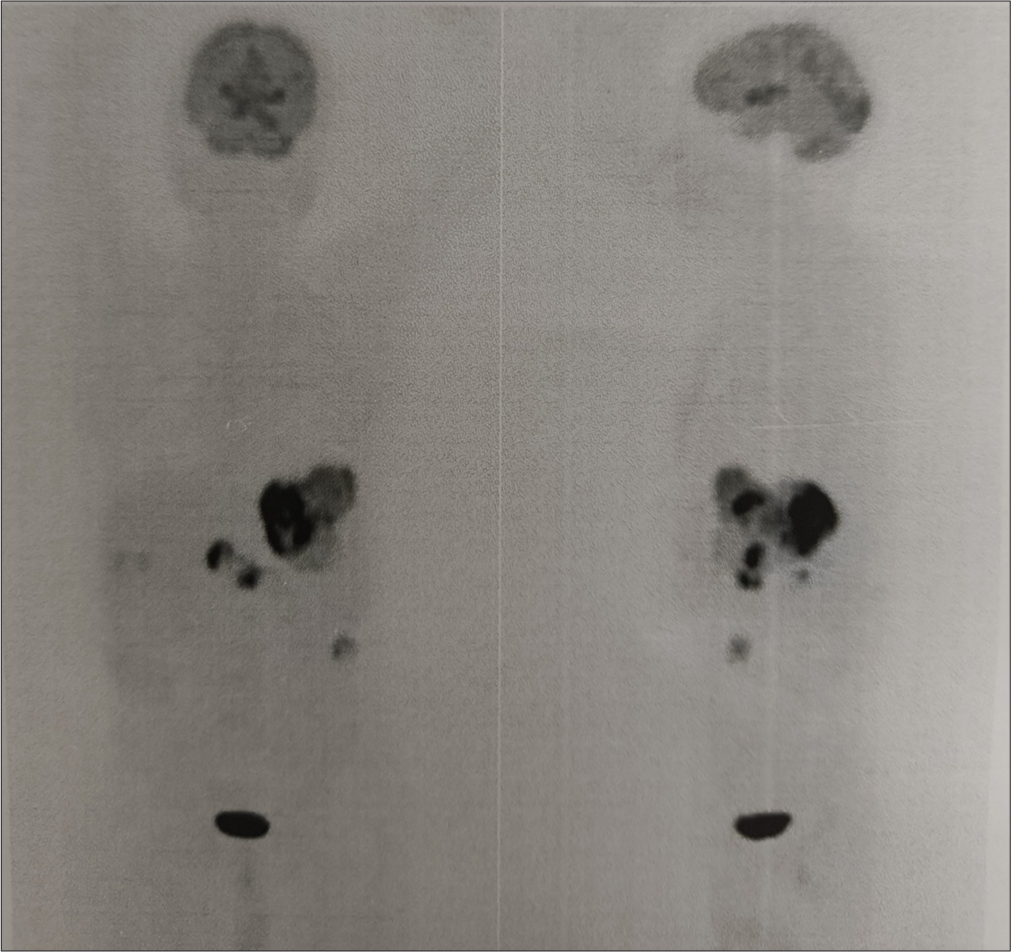
- Pre-therapy fluorodeoxy glucose (FDG) positron emission tomography scan of the patient with hepatocellular carcinoma showing a large disease in the left lobe of liver with para-aortic nodes.
He was started on standard doses of atezolizumab (anti-programmed cell death ligand 1) with bevacizumab (anti-VEGF); he completed 2 cycles (at 3 weekly intervals) on September 24, 2024. On October 04, 2024, he presented to the emergency department with acute hematemesis. An esophagoscopy showed a large ulcerating growth in the fundus of the stomach with active bleeding and grade I varices. He underwent embolization of the large infiltrating hepatic tumor of the left lobe on October 10, 2024. He was later discharged in stable condition. On October 12, 2024, he again presented to the ER with acute breathlessness, hypoxia, and hypotension. A chest X-ray showed cardiomegaly with bilateral pulmonary edema. An emergency 2D echocardiography showed an EF of only 25% with biventricular hypokinesia. The serum troponin I was 0.911 ng/mL (normal: 0–0.04 ng/mL) and pro-B-type natriuretic peptide (BNP) was >30000 pg/mL (Normal <300 pg/mL). The hemogram showed a hemoglobin 10.3 g/dL, total count of 21800/cmm, and normal platelets. He had no other muscular weakness.
After a cardiologist’s opinion, he was presumed to have acute ICI-induced myocarditis (ICI-myocarditis) and started on high-dose methylprednisolone 1000 mg/day along with other cardiac support and diuretics. Within 4–5 h, there was a dramatic improvement in his condition including tachycardia, hypotension, and hypoxia. After 3 days of methylprednisolone, the pro-BNP was reduced to 14000 pg/mL. The pulmonary congestion was less on chest X-ray and there was a marginal improvement (EF 30%) in the EF on 2D echocardiography. He was shifted to the ward on the 5th day and was off oxygen or inotropic support.
DISCUSSION
ICI myocarditis is one of the rare complications of an ICI with an incidence of 0.09–1.5%.[1,2] In a single institute study in which ICI was given to 964 patients between 2013 and 2017, 11 patients (1.14%) developed myocarditis.[3] The incidence of cardiotoxicities is higher with dual ICI like nivolumab plus ipilimumab than single agent.[4 -7] A combination of ICI (atezolizumab) and an anti-VEGF inhibitor (bevacizumab) is now a standard of care in advanced hepatocellular carcinoma. Another meta-analysis showed that the combination has potentially more cardiac and blood-related toxicities than single-agent bevacizumab.[8] The International CardioOncology Society has defined five types of cardiac toxicities associated with cardiotoxic drugs – heart failure, vascular toxicities, hypertension, myocarditis, and arrhythmias.[9] The diagnosis is many times missed as the symptoms of early toxicity are non-specific such as fatigue, breathlessness on exertion, hypertension, and giddiness. The diagnosis is one of exclusion after excluding primary coronary disease, which has similar features. ICI-induced myocarditis is usually defined by Bonaca’s criteria and includes the clinical features, ECG changes, raised cardiac enzymes, 2D echocardiography with a confirmatory diagnosis by cardiac magnetic resonance (CMR) and endomyocardial biopsy.[10] We could not do a biopsy because the patient’s condition did not allow us to do it. Myocarditis is categorized into three types – definite, possible, and probable myocarditis. A definitive diagnosis is based on endomyocardial biopsy, a CMR imaging with ECG changes and elevated cardiac biomarkers (Troponin and proBNP), and a new cardiac wall motion abnormality on 2D echocardiography with ECG changes and raised biomarkers.
An algorithm based on troponin and creatinine phosphokinase (CPK) levels has been proposed for the diagnosis of ICI myocarditis. It divides patients receiving ICIs with elevated troponins and symptoms of myocarditis into those that develop symptoms within 60 days of the first ICI infusion and those that develop beyond 60 days of the first infusion. The group developing symptoms beyond 60 days of the infusion with normal CPK levels and reducing troponins are least likely to have ICI-induced myocarditis.[11,12] The group developing symptoms within 60 days of infusion with elevated CPK and troponins has a high probability of having ICI-induced myocarditis.
The etiopathogenesis of ICI-induced myocarditis is not clear. However, it involves an inflammatory response with infiltration of the myocardium with macrophages and CD4+ and CD8+ T-lymphocytes. The T cells attack the antigens present on the tumor, skeletal muscles, and heart tissues.[13] ICI myocarditis usually develops early with the initiation of ICI therapy. The median time to occurrence was between 3 and 4 weeks (after 2nd dose of the drug), though cases are reported occurring even after the first dose of the immunotherapy.[14] Our patient presented with myocarditis after 6 weeks of starting the ICI drug. Hence, it is an early-onset irAE as against hypothyroidism or gastrointestinal toxicity which occurs much later. Patients developing cardiotoxicity within the 1st year of treatment were more likely to have other irAEs than patients developing cardiotoxicity after 1 year.[5]
Myocarditis is sometimes associated with myositis and myasthenia due to ICI therapy. Such patients present with typical features of myocarditis along with muscle pains and symptoms of myasthenia gravis. As mentioned previously, the incidence of ICI-induced myocarditis is 0.09–1.5% and among these, 25% of patients also have myositis and 11% approximately have myasthenia too. This is known as the myocarditis-myositis-myasthenia (MMM) overlap syndrome.[15,16]
The mortality due to severe ICI-induced myocarditis is as high as 50%.[5,17,18] In the pharmacovigilance data of the World Health Organization, of 122 patients with ICI-induced myocarditis, 61 (50%) died during a median follow-up of 180 days.[2] There are different grades of ICI-induced myocarditis as shown in Table 1.
| Grade of myocarditis | Clinical features |
|---|---|
| Grade 1 | Asymptomatic or abnormal rise in biomarkers or ECG changes |
| Grade 2 | Mild symptoms with abnormal biomarkers or abnormal ECG |
| Grade 3 | Reduced wall motion or LVEF; positive or suggestive features on cardiac MRI |
| Grade 4 | Life-threatening condition with all features from grade 1 to 3 |
ICI: Immune checkpoint inhibitor, ECG: Electrocardiogram, MRI: Magnetic resonance imaging, LVEF: Left ventricular ejection fraction
ICI-induced myocarditis many times develops insidiously and a high index of suspicion is needed to detect it early on and prevent fatalities. Subtle changes in ECG and 2D echocardiography can identify patients developing early myocarditis.[19] This could potentially prevent severe and fatal myocarditis and have a positive impact on quality of life, not to mention the cost saving of treating patients with severe myocarditis and heart failure in the critical care unit.[20] Gao et al. in their study found that nearly 72% patients had ECG abnormalities at the start of myocarditis, with ST-T abnormalities (47.9%) being the most common among these patients, followed by QTc interval prolongation (31.5%).[19] Sinus arrhythmias were found in 26%. In the patients with severe myocarditis, 90% showed ECG abnormalities with the onset of myocarditis. The most common change in this group was QTc prolongation (70%) and sinus tachycardia (50%). Overall, the four most common ECG abnormalities found were sinus tachycardia, prolonged QRS to more than 110 ms, prolonged QTc interval, and bundle branch block. These changes early in the course of treatment with ICI could predict severe myocarditis. The European Society of Cardiology guidelines recommend doing an ECG and troponins at the start of every cycle of immunotherapy for the first 4 cycles and then every 3rd cycle.[21]
The primary treatment of any patient suspected to have ICI-induced myocarditis (new ECG changes, new sinus tachycardia, bundle branch blocks, or recent elevated troponins) is to stop the immunotherapy agent. In case of severe or fulminant myocarditis, patients should be admitted to the intensive care unit with ECG monitoring and started with high doses of methylprednisolone (1000 mg/day) for 5 days and then shifted to oral prednisolone (1 mg/kg/day).[22,23] Regular monitoring of cardiac enzymes, 2D echocardiography, and clinical parameters should be done. The response rate to initial methylprednisolone is seen in approximately 50% of patients;[24] those that do not respond are considered steroid refractory and offered second-line therapies such as intravenous immunoglobulins, plasmapheresis, mycophenolate mofetil, alemtuzumab (anti-CD52 antibody), and tofacitinib. Our patient responded dramatically to the first two doses of high-dose methylprednisolone with a rapid reduction in pulse rate, oxygen requirements, and a reduction in the proBNP and troponin levels. He however succumbed to the toxicity at home after 40 days. As a general rule, patients with ICI-induced myocarditis are not rechallenged with the immunotherapy drug.
Patients on chemotherapy and ICIs can be offered protection from heart failure with sodium-glucose co-transporter 2 inhibitors (inhibitors) such as dapagliflozin and empagliflozin. A recent publication has suggested the role of these agents in protecting from heart failure due to chemotherapy and ICIs.[25,26]
CONCLUSION
With the increasing use of ICIs in the treatment of various cancers, the incidence of immune-related adverse events is on the rise. ICI-induced myocarditis can occur in isolation, but also as a MMM overlap syndrome. It is an early irAE, occurring usually within the first 30–40 days of initiating the therapy. As over half of the patients with toxicity die within 2 months, a high index of suspicion and frequent monitoring of ECG and cardiac enzymes is needed to detect early toxicity and prevent fulminant myocarditis. The mainstay of treatment is early initiation of high doses of steroids. The real cost of ICI therapy needs to be elucidated with a cost-benefit analysis taking into consideration the costs of treating the toxicities.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
Shirish Alurkar is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018;4:1721-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579-8.
- [CrossRef] [PubMed] [Google Scholar]
- Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755-64.
- [CrossRef] [PubMed] [Google Scholar]
- Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749-55.
- [CrossRef] [PubMed] [Google Scholar]
- Immune checkpoint inhibitors-associated cardiotoxicity. Cancers (Basel). 2022;14:1145.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiotoxicity associated with immune checkpoint inhibitor therapy: A meta-analysis. Eur J Heart Failure. 2021;23:1739-47.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiotoxicity of immune checkpoint inhibitors: A frequency network meta-analysis. Front Immunol. 2022;13:1006860.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing cardiovascular adverse events in cancer patients: A meta-analysis of combination therapy with angiogenesis inhibitors and immune checkpoint inhibitors versus angiogenesis inhibitors alone. Crit Rev Oncol Hematol. 2023;188:104059.
- [CrossRef] [PubMed] [Google Scholar]
- Defining cardiovascular toxicities of cancer therapies: An International cardio-oncology society (IC-OS) consensus statement. Eur Heart J. 2022;43:280-99.
- [CrossRef] [PubMed] [Google Scholar]
- Myocarditis in the setting of cancer therapeutics: Proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140:80-91.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular adverse events associated with immune checkpoint inhibitors: A retrospective multicenter cohort study. Cancer Med. 2024;13:e7233.
- [CrossRef] [PubMed] [Google Scholar]
- Role of biomarkers in the management of immune-checkpoint inhibitor-related myocarditis. Curr Cardiol Rep. 2023;25:959-67.
- [CrossRef] [PubMed] [Google Scholar]
- New-onset third-degree atrioventricular block because of autoimmune-induced myositis under treatment with anti-programmed cell death-1 (nivolumab) for metastatic melanoma. Melanoma Res. 2017;27:155-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiotoxicity induced by immune checkpoint inhibitors: A pharmacovigilance study from 2014 to 2019 based on FAERS. Front Pharmacol. 2021;12:616505.
- [CrossRef] [PubMed] [Google Scholar]
- Myocarditis, myositis, and myasthenia gravis overlap syndrome associated with immune checkpoint inhibitors: A systematic review. Diagnostics (Basel). 2024;14:1794.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring pembrolizumab-induced myocarditis, myositis, and myasthenia gravis: A comprehensive literature review and case presentation on bladder cancer. Cureus. 2023;15:e49867.
- [CrossRef] [Google Scholar]
- Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085-7.
- [CrossRef] [PubMed] [Google Scholar]
- A comprehensive review on immune checkpoint inhibitors induced cardiotoxicity characteristics and associated factors. Eur J Med Res. 2023;28:495.
- [CrossRef] [PubMed] [Google Scholar]
- Early identification of severe immune checkpoint inhibitor associated myocarditis: From an electrocardiographic perspective. Cancer Med. 2024;13:e7460.
- [CrossRef] [PubMed] [Google Scholar]
- The Impact of adverse events on health care resource utilization, costs, and mortality among patients treated with immune checkpoint inhibitors. Oncologist. 2021;26:e1205-1.
- [CrossRef] [PubMed] [Google Scholar]
- 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the International cardio-oncology society (IC-OS) Eur Heart J Cardiovasc Imaging. 2022;23:e333-465.
- [Google Scholar]
- Society for immunotherapy of cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9:e002435.
- [CrossRef] [PubMed] [Google Scholar]
- Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141:2031-4.
- [CrossRef] [PubMed] [Google Scholar]
- Immune checkpoint inhibitor-associated myocarditis: A systematic analysis of case reports. Front Immunol. 2023;4:1275254.
- [CrossRef] [PubMed] [Google Scholar]
- The cardioprotective and anticancer effects of SGLT2 inhibitors: JACC: Cardiooncology State-of-art-review. JACC CardioOncol. 2024;6:159-82.
- [CrossRef] [PubMed] [Google Scholar]
- Sodium-glucose co-transporter-2 inhibitors in patients treated with immune checkpoint inhibitors. Cardiooncology. 2024;10:2.
- [CrossRef] [PubMed] [Google Scholar]







