Translate this page into:
Role of stem cell transplant in chronic myeloid leukemia in contemporary settings

*Corresponding author: Shailesh Pravinsinh Lavana, Department of Hematology and Stem Cell Trasplant, Kailash Cancer Hospital and Research Centre, Goraj, Vadodara, Gujarat, India. drslavana@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Lavana SP. Role of stem cell transplant in chronic myeloid leukemia in contemporary settings. Int J Mol Immuno Oncol. 2025;10:23-31. doi: 10.25259/IJMIO_15_2024
Abstract
The discovery of “magic bullet oral tyrosine kinase inhibitors (TKIs)” has revolutionized the treatment landscape for chronic myeloid leukemia (CML) and supplanted the use of allogeneic hematopoietic stem cell transplant (SCT) as a first-line treatment. Similar trends were observed in India also. Despite the remarkable improvement in outcomes of chronic phase CML, therapeutic challenges persist demanding alternative therapy for patients encountering tyrosin kinase resistance or intolerance. Allogeneic hematopoietic stem cell transplant (HSCT) retains its significance, especially in refractory and intolerant cases, with careful consideration of the patient’s specific factors and disease phases. The optimal timing of allogeneic SCT remains crucial as the disease phase exerts a substantial impact on outcomes. Allogeneic HSCT is cost cost-effective, viable, and potential curative option for managing T315I mutant CML in resource-constrained countries like India. Allogeneic HSCT is an essential therapeutic option, offering long-term remission and disease control compared to TKI alone in an advanced phase of CML. Moreover, pre-transplant TKI therapy exhibits safety and improves post-transplant survival outcomes, while post-transplant TKI maintenance therapy decreases relapsed rate. It is advisable to conduct regular monitoring using sensitive techniques for an indefinite period following transplantation. These insights stress the imperative of tailored therapeutic strategies and vigilant monitoring to optimize outcomes for CML patients in contemporary clinical settings.
Keywords
Advanced phase of chronic myeloid leukemia
Allogeneic hematopoietic stem cell transplant
Chronic myeloid leukemia
Cost effective
Tyrosine kinase inhibitor resistant or intolerant
CML INTRODUCTION
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder resulting from the transformation of hematopoietic stem cells by the BCR-ABL fusion gene. Most of the patients present in the chronic phase (CP). Without appropriate treatment, it usually transforms from a CP to an accelerated and blasts phase, which are typically fatal.
The decision regarding undergoing an allogeneic hematopoietic stem cell transplant (HSCT) for the hematological disorder is intricate. It depends on various factors, including predicted outcomes with available non-transplant therapy compared to stem cell transplant (SCT).
Historically, treatment options were limited for CML and HSCT is the only curative option.[1-3] However, worldwide treatment algorithms drastically changed with the advent of “magic bullet-oral tyrosine kinase inhibitors” targeting the specific abnormality in CML cells. Tyrosine kinase inhibitor (TKI) therapy particularly in the CP resulted in impressive long-term survival rates without immediate treatment-related mortality, thus surpassing HSCT as the preferred first-line treatment, especially in CP.[1-3] Consequently, worldwide the use of HSCT has declined over time in the CP [Figure 1].[2] This similar trend is mirrored in India with the availability of imatinib through a sponsored program.[4,5] The data from Indian Society for Blood and Marrow Transplantation evident this shift by accounting for only a small fraction of allogeneic SCTs for CML of all allogeneic SCTs [Figure 2].[6]
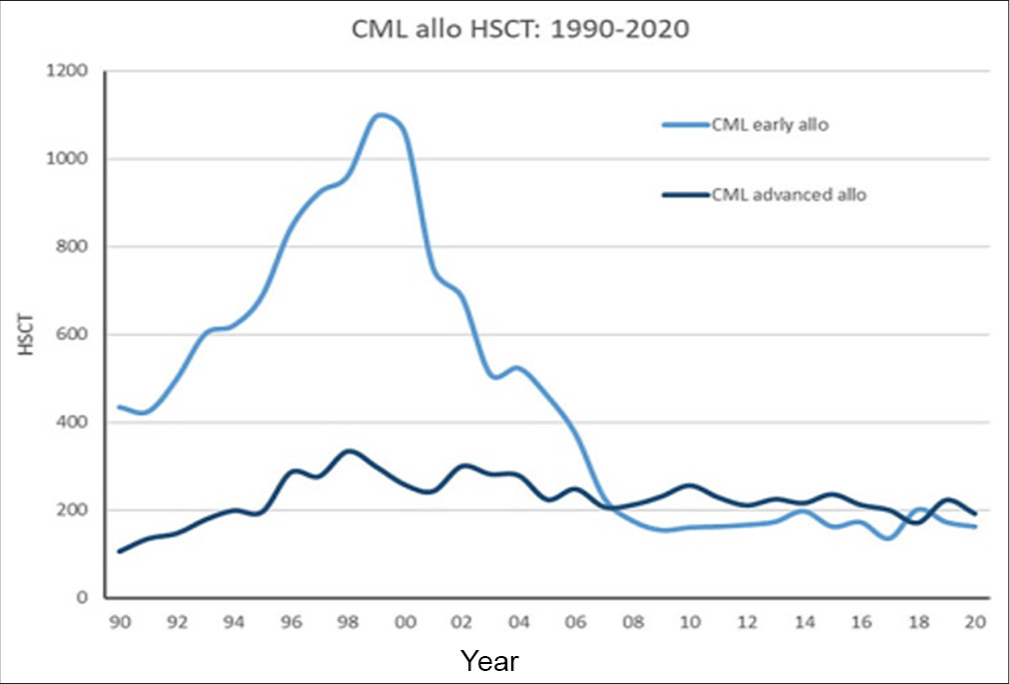
- Allogeneic stem cell transplantation for chronic myeloid leukemia (CML) from 1990 to 2020 (European Society for Blood and Marrow Transplantation registry) showing chronic phase and advanced phase CML (accelerated phase and blast crisis). HSCT: Hematopoietic stem cell transplant.
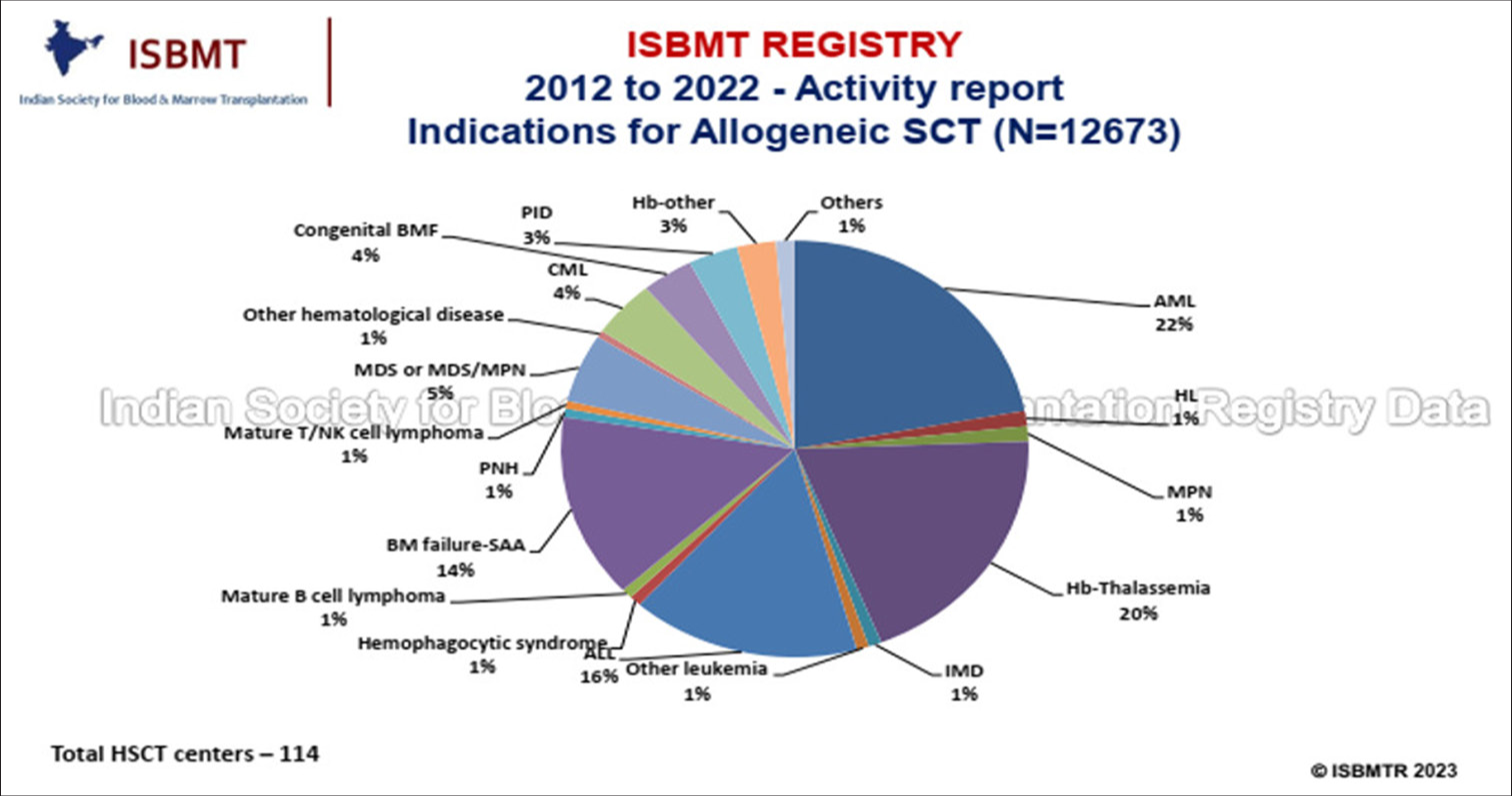
- Indications for allogeneic stem cell transplant 2012 to 2022 – Activity report (Indian Society for Blood and Marrow Transplantation Registry). AML: Acute myeloid leukemia, HL: Hodgkin’s lymphoma, MPN: Myeloproliferative neoplasm, Hb: Haemoglobinopathies, IMD: Inherited metabolic disease, CML: Chronic myeloid leukemia, SAA: Severe aplastic anemia, MDS: Myelo dysplastic syndrome, PID: Primary immuno deficeiency, PNH: Paroxysmal nocturnal hemoglobinuria, BMF: Bone marrow failure, ISBMT: Indian society for blood and marrow transplantation, NK: Natural killer cells.
In 2010, the concept of “Operational cure” was introduced and imatinib can be discontinued provided a stable complete molecular response which has been obtained.[7] Despite these advancements, therapeutic challenges persist for a significant proportion of patients and demand alternative therapy for patients experiencing treatment resistance or intolerance.
The outcomes of HSCT have also improved significantly over time. However, the effect of the disease phase on outcome has not changed over the years [Figure 3], with transplant-related mortality rates ranging from 5% to 10% in CP CML and 20–25% in the advanced phase.[8] Hence, the timing of HSCT remains critical, as undergoing transplantation too early or too late can impact outcomes.
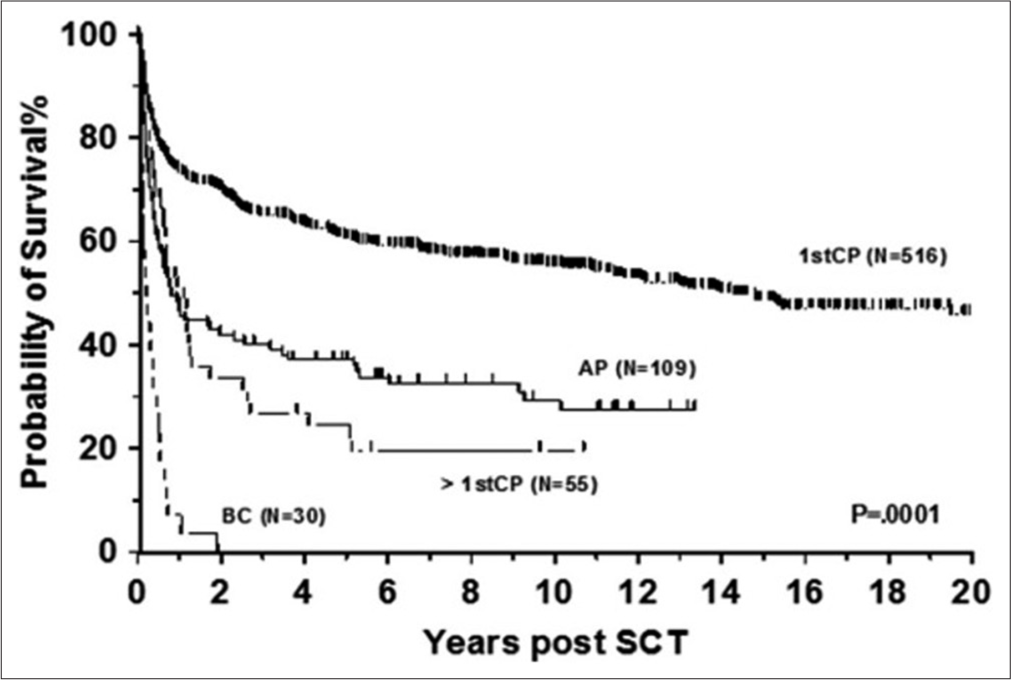
- Allogeneic stem cell transplantation for chronic myeloid leukemia in various phase. AP: Accelerated phase, CP: Chronic phase, BC: Blast crisis, SCT: Stem cell transplant.
In summary, TKIs have transformed the treatment landscape for CML and reduced the need for HSCT as a first-line therapy. However, HSCT is still a curative option for patients with CML, but decisions for the same require careful consideration of individual factors and disease status in the present era. Understanding when HSCT may be the most suitable option and how TKI can be integrated into pre- and post-transplant settings is essential for optimizing outcomes in CML.
CML-CP
The decision to pursue allogeneic SCT in patients with CML-CP is complex. Oral TKI therapy has significantly elevated overall survival (OS) rates in these patients, with negligible immediate drug-related mortality. Consequently, TKIs have displaced SCTs as the primary first-line therapy for CML.
Achieving specific milestones in response to TKI treatment, such as complete cytogenetic response, early molecular response, and major molecular response are indeed valuable surrogate markers for long-term progression-free survival (PFS) and OS in patient CML-CP.[9-11]
Frontline therapy with imatinib yields a major molecular response in approximately 60% of CP patients, while dasatinib and nilotinib boast even higher efficacy rates, achieving major molecular responses in around 75% of patients at 4–5-year follow-ups.[12-14] The 10-year OS with imatinib is approximately 85%.
A study from Tata Memorial Hospital, Mumbai (India) reported complete cytogenetic response in 77% of patients with front line TKI therapy with imatinib.[15] However, another study from India, Ganesan et al. have reported the significantly lower 10-year OS with Imatinib, which is around 75% and attributed to low adherence to imatinib.[4]
A substantial proportion – 25–40% – in western country may exhibit either drug intolerance or resistance, necessitating a transition to second-line therapy.[12-14] Similarly, a study from Tata memorial hospital, Mumbai (India) also documented resistance or relapse in 38% of patients treated with frontline TKI imatinib.[15] German CML-registry (CML-VI) has reported a TKI switch of 31% after a median of 11 (0-68) months. Reasons for switching were failure in 32%, intolerance in 57%, and 11% switched TKI due to other reasons.[16]
In cases of imatinib failure, second-generation TKIs (2GTKIs) such as nilotinib or dasatinib achieve complete cytogenetic responses in approximately 40–45% of patients, indicating a significant need for third-line treatment.[17,18] One study from India reported that only 30% achieve major molecular response with nilotinib as a second-line treatment,[19] suggesting that a significant number of patients will require third-line treatment.
Milojkovic et al. have developed a scoring system to predict cytogenetic response with 2GTKIs, aiding in identifying patients less likely to respond and requiring alternative therapies. This scoring system includes the cytogenetic response to imatinib, Sokal score, and recurrent neutropenia during imatinib treatment.[20]
Third-line treatment with second-generation drugs is associated with limited efficacy, achieving complete cytogenetic responses in only 20–30% of patients, with uncertain response durability.[21] In case of 2G TKI failure or intolerance, ponatinib as third-line treatment achieves complete cytogenetic response and MMR in approximately 50% and 40% of patients, respectively.[22] Similarly, asciminib achieves comparable responses in patients previously treated with multiple TKIs.[23] However, the availability and affordability of these advanced therapies remain significant challenges, especially in developing countries like India.
Despite advancements in TKI therapy, data suggest that a substantial minority of patients (~10–15% in Western countries) may not achieve an optimal response and may require alternative strategies such as allogeneic HSCT.[24] In India, this proportion may increase to approximately 20–30% due to cost and availability issues with ponatinib or asciminib [Figure 4].
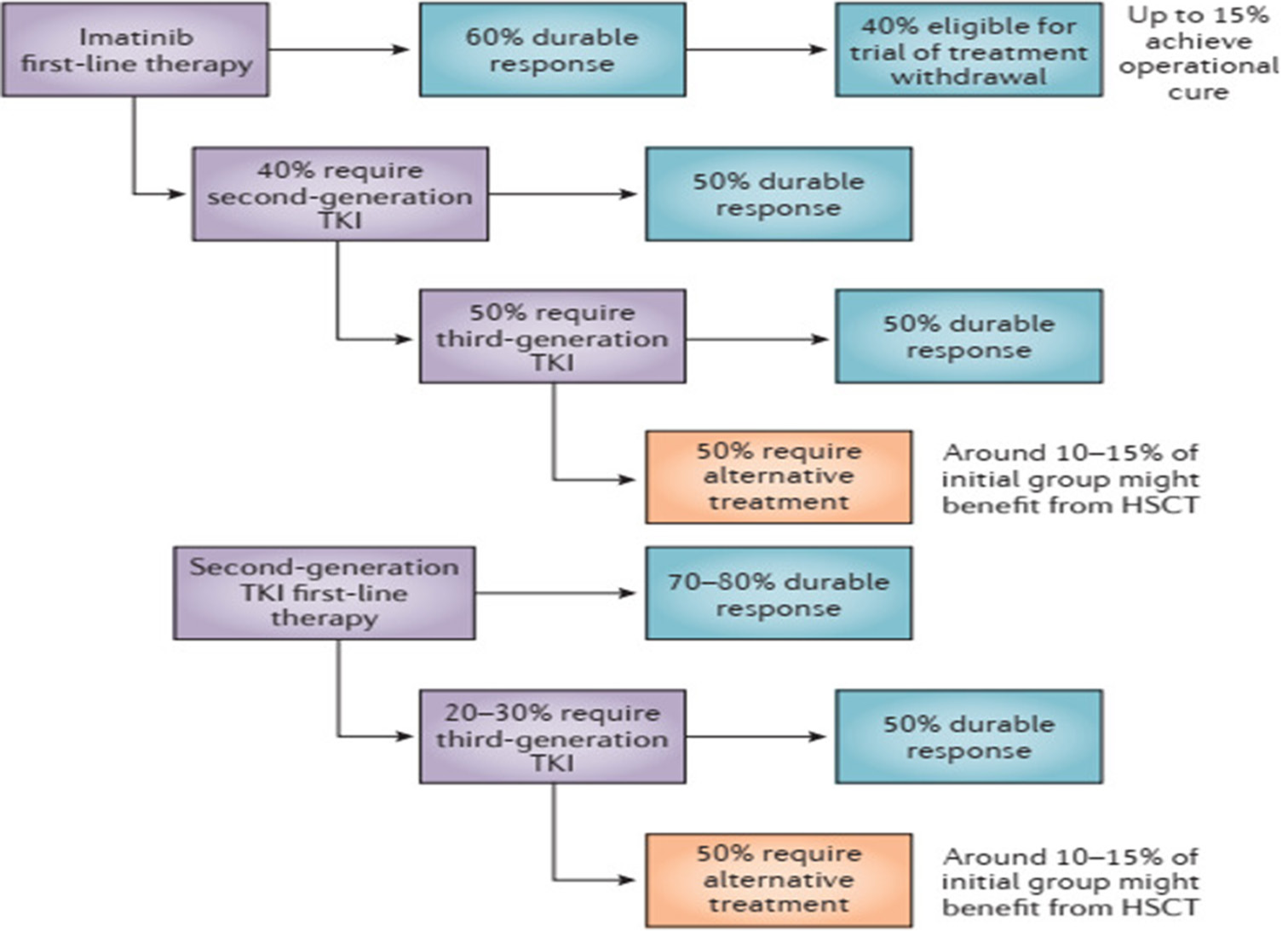
- Overall response rates to tyrosine kinase inhibitor in chronic myeloid leukemia-chronic phase. TKI: Tyrosin kinase inhibitors, HSCT: Hematopoetic and stem cell transplant.
Even though a rare adverse event, some patients may develop bone marrow aplasia following TKI exposure, without significant reduction in CML clone. These patients are not amenable to TKI treatment, in fact necessitating interruption of TKI and sometimes requiring cytokine support.[25] For patients who are unlikely to regain normal hematopoiesis, allogeneic HSCT represents a potentially curative approach. The donor healthy stem cell can restore normal hematopoiesis and potentially treat both CML- and TKI-induced bone marrow aplasia.
The T315I mutation is one of most frequent (20%) mutation found in TKI resistant patients and serves as a primary driver of resistance to both first (1G) an second (2G) TKI inhibitors.[26,27] While promising, agents such as ponatinib and asciminib have shown efficacy in overcoming this resistance, practical challenges such as the need for lifelong treatment, high costs, and limited drug accessibility present significant hurdles. In resource-constrained settings like India, where financial constraints and drug availability issues are prevalent, alternative strategies such as allogeneic SCT emerge as viable and potentially curative options for managing T315I-mutant CML.
TRANSPLANT OUTCOME IN CML-CP1
Transplant outcomes have improved over the past three decades, with low transplant-related mortality rates (8%) compared to earlier studies.[8,28] Many retrospective and single-center studies report 3-year OS 70–90% with allogeneic SCT as second-line therapy after imatinib failure[28-30] [Table 1].
| Study | Patients (n) | CML-CP (%) | 5 years OS (%) |
|---|---|---|---|
| Chaudhury et al.[30] | 449 177 (<18 year) 272 (18–29 year) |
100% | 75% |
| Yassine et al.[29] | 187 (Adults) 23 (Pediatric/Adult) 164 (Mixed) |
100% | 84% (Adults) 91% (Pediatric) 82% (Mixed) |
| Parthiban et al.[31] (India) | 40 | 65% | 70% |
CML-CP: Chronic myeloid leukemia chronic phase, OS: Overall survival, SCT: Stem cell transplant
Similarly, in India, Parthiban et al., have reported 70% OS with allogeneic SCT in CML patients with TKI failure. However, non-relapse mortality was high (20%) in their cohort.[31]
COST OF SCT VERSUS TKI
Comparing the costs, an allogeneic SCT in India typically ranges from INR ₹9 to 35 lakhs, with an average of around ₹15 lakhs.[32] On the other hand, the monthly cost of asciminib is approximately ₹2,35,000 inclusive GST, and the cost of ponatinib is even higher. Despite the higher initial investment, SCT emerges as a cost-effective treatment option with acceptable outcomes.
CML ACCELERATED PHASE (CML-AP)
The introduction of TKI has modified the natural history of CML and significantly decreased the number of CM-AP. The monitoring milestones are not well defined like the CP. However, monitoring with BCR-ABL1 transcripts is recommended at 3-month intervals, similar to CM-CP. Failure to achieve these milestones, as observed in CP-CML, should prompt consideration of changing therapy and potentially proceeding to hematopoietic stem cell transplantation.[12]
The timing of transplantation is particularly challenging for patients who present de novo with AP-CML. Jiang et al. demonstrated a significant advantage of allogeneic HSCT for high and intermediate-risk patients with AP-CML compared to imatinib, while outcomes of both therapies are equally favorable in low-risk patients.[33] They have identified three significant independent adverse factors for OS and PFS: Hemoglobin <10 g/dL, peripheral blasts exceeding 5%, and CML duration greater than 12 months. These factors can help to guide treatment decisions and risk stratification in CML-AP patients.
CML BLASTS PHASE/CRISIS (CML-BP/BC)
The BP/BC in CML presents a stark contrast to the CP, bearing fatal consequences if left untreated. Despite a decline in its incidence during the TKI era, the response to TKI in blasts crisis remains fleeting. While median survival has improved in the TKI era (7–11 months) compared to the preTKI era (3–4 months),[34] it falls short of satisfaction without a SCT. Various studies have shown better OS with SCT in CML-BC [Figure 5].[35] Enter HSCT – the beacon of hope for long-term survival in this cohort and given the transient nature of responses post-TKI or chemotherapy, prompt initiation of donor search on blasts crisis diagnosis becomes paramount.
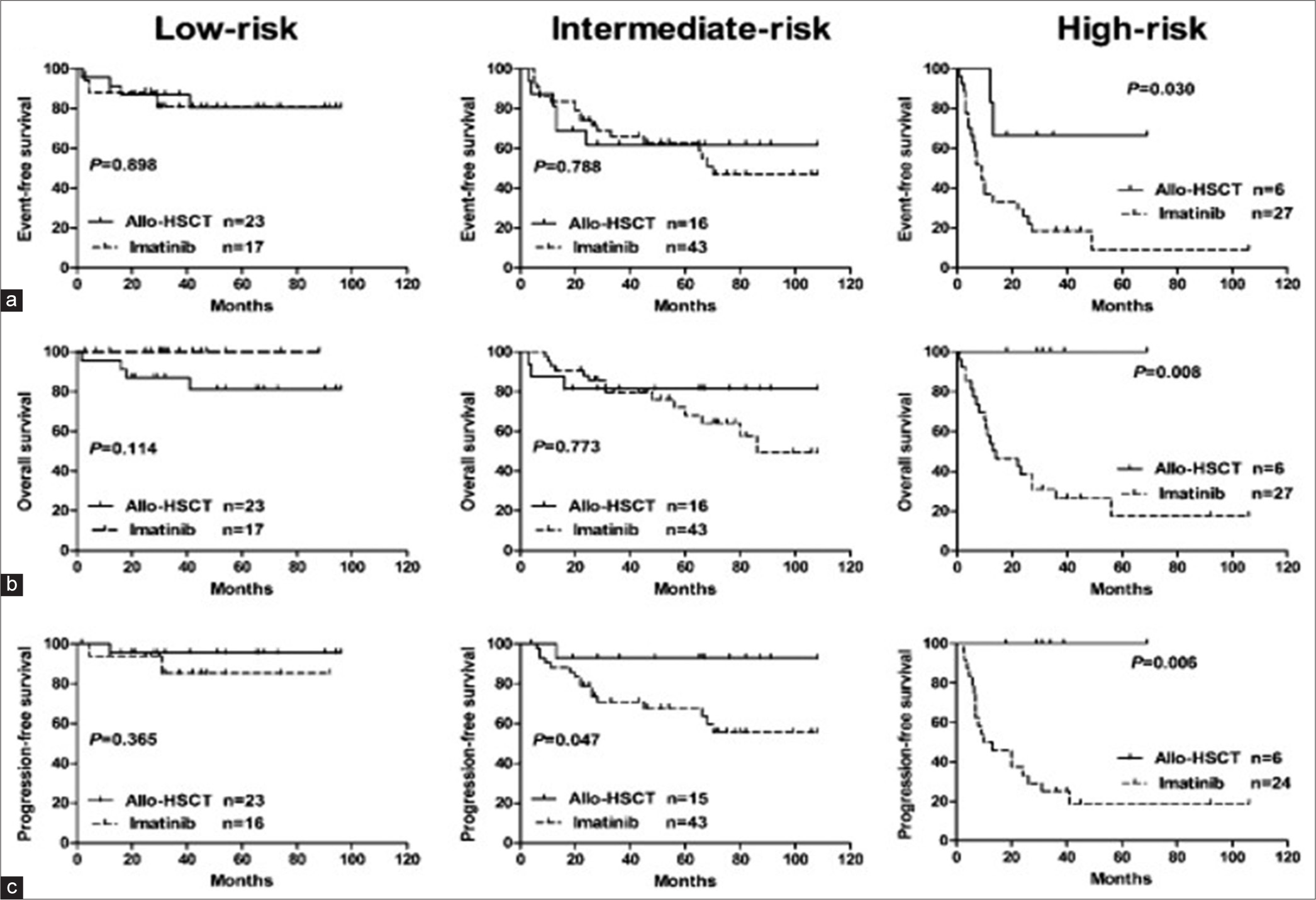
- (a-c) Outcome of chronic myeloid leukemia accelerated phase, imatinib versus allo-allogeneic hematopoietic stem cell transplant. HSCT: Hematopoietic stem cell transplant
The objective is to attain the second CP or remission, as for those undergoing allo-SCT during active BC, the odds of OS and leukemia-free survival (LFS) post-transplant are significantly lower compared to those grafted during remission [Figure 6].[36] Enter the second CP, attainable through TKI, intensive chemotherapy, or a synergistic blend of both. Studies have shown that incorporating a TKI into chemotherapy-based regimens significantly enhances the likelihood of achieving a complete hematologic response (CHR) and transitioning to the second CP.[35,37] For lymphoid blasts crisis, nearly 90% of patients achieve CHR with the combination, compared to only 35% with TKI alone.[35,38] Similarly, in myeloid blast crisis, the combination yields complete hematological response rates of 50–60%, in contrast to 10–25% with TKI monotherapy.[39] European leukemia net and National comprehensive cancer network guideline also recommends TKI plus chemotherapy to achieve a second CP before transplant. The choice of TKI hinges on mutation analysis and prior TKI exposure.
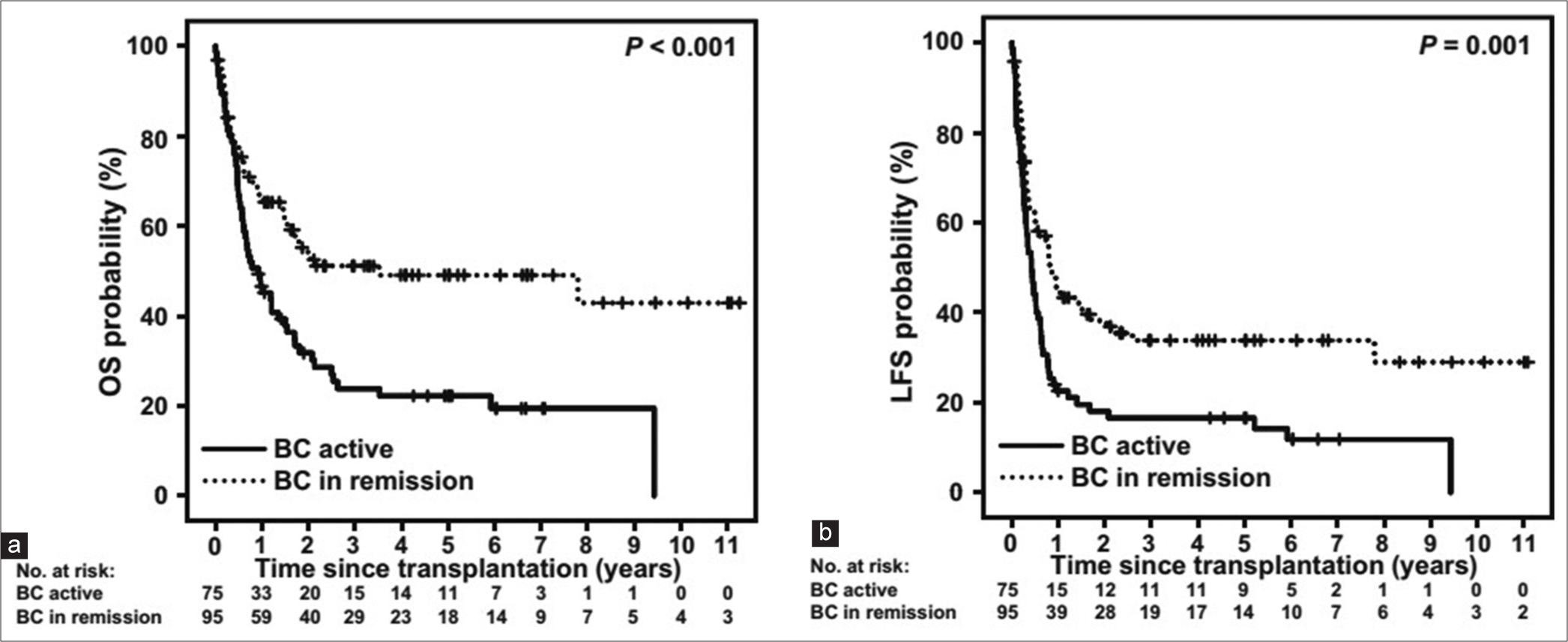
- (a and b) Outcome of allogeneic stem cell transplantation for chronic myeloid leukemia in blast crisis. OS: Overall survival, LFS: Leukemia free survival, BC: Blast crisis.
In a study conducted in India by Nakka et al., the 2-year LFS and OS rates after allogeneic SCT in CML blast crisis were found to be 43% and 57%, respectively.[40,41] These findings align closely with the other studies [Table 2] and the largest series of transplant outcomes reported in the literature for the CML blast crisis. Specifically, data from the Center for International Blood and Marrow Transplant Research (CIBMTR) Registry and the European Society for Blood and Marrow Transplantation Registry indicate a three-year survival rate of approximately 40%.[42] This suggests that SCT holds promise as a viable treatment option for individuals facing CML blast crisis, with comparable survival outcomes observed across different cohorts and regions.
| Study | Patients (n) | CML-BC | Relapse (%) | 3 years OS (%) | Treatment-related mortality (%) |
|---|---|---|---|---|---|
| Nicolini et al.[41] | 63 | 24 | 40 | 36 | 33 |
| Khoury et al.[42] | 449 | 80 | 36 | 14 | 54 |
| Radujkovic et al.[36] | 170 | 170 | 51 | 39 | 23 |
| Niederwieser and Kröger[2] | 147 | 97 | 43 | 34 | 28 |
| Nakka et al.[40] (India) | 48 | 7 | 43 | ear | ear |
CML: Chronic myeloid leukemia, BC: Blast crisis, SCT: Stem cell transplant, OS: Overall survival
There were no relapses beyond 5 years post-SCT despite a 43% cumulative incidence of relapse underscores the effectiveness of SCT in providing durable remission and long-term disease control.[43] This finding supports the role of SCT as a valuable treatment option for leukemia patients aiming for sustained remission. The lack of studies from India on the long-term outcomes of transplantation in advanced-phase CML highlights a gap in research and clinical knowledge in this area.
CML PRE TRANSPLANT TKI
Initially, there was concern that the use of TKI as the first line may increase transplant-related mortality. However, subsequent investigation may have demonstrated the safety of both first and second-generation TKIS when administered before stem cell transplantation.[44,45] In addition, emerging case reports have suggested the safety of third-generation TKI such as ponatinib in pre-transplant settings.[46]
Pre-transplantation TKI therapy has been associated with favorable post-transplant outcomes. Notably, Oehler et al. observed a correlation between achieving a major or complete cytogenetic response to TKI and a reduced hazard of transplant-related mortality.[47]
Conversely, a single-center study conducted in India reported an increased risk of thrombotic microangiopathy associated with pre-transplant TKI use.[48] Nevertheless, further prospective studies are required to corroborate these findings and ascertain causal relationships.
POST-TRANSPLANT MONITORING
The CIBMTR data indicate a cumulative incidence of relapse at 15 years for patients maintaining remission at 5 years post-transplantation with a matched sibling donor which is 17%.[49] Therefore, it is advisable to conduct regular monitoring every 3–6 months using sensitive techniques for an indefinite period following stem cell transplantation.
Early detection of the BCR/ABL1 transcript shortly after transplantation lacks clinical significance. However, the status of the BCR/ABL1 transcript (persistently negative, fluctuating, and persistently positive) at 6 months post-transplantation predicts the likelihood of relapse.[49]
Kaeda et al. defined criteria for molecular relapse post-transplantation is by either three polymerase chain reaction (PCR) results over a minimum period of 4 weeks with a BCR-ABL/ABL ratio exceeding 0.02%, or two results higher than 0.05% over a minimum period of 4 weeks. They found the projected incidence of cytogenetic and hematological relapse after 5 years from the diagnosis of molecular relapse is estimated at 70%.[49]
POST-TRANSPLANT MAINTENANCE TKI
A cohort analysis revealed promising 5-year OS rates of 79% among patients with advanced phase CML receiving TKI maintenance therapy post-transplantation, along with decreased relapse rate.[50] These findings emphasize potential survival benefits associated with TKI maintenance in post-transplantation management of CML. However, the optimal timing to start, dose, and duration of administration of TKIs post-transplantation remains to be defined.
TKI maintenance therapy typically commences around day +100 post-transplantation, however, they can be safely administered earlier. Importantly, initiating TKI before 90 days post-transplantation has been considered safe and effective, underscoring the potential for early intervention.[51]
Carpenter et al. have reported that patients may tolerate post-transplant Imatinib at a standard dose intensity-like primary therapy. In their study, adult tolerated a median average daily dose of imatinib of 400 mg/day (range, 200–500 mg/day), while children tolerated 265 mg/m2/day (range, 200–290 mg/m2/day). Second-generation TKI should be initiated at 50% of the dose before transplantation.[51]
The optimal duration of TKI therapy post-transplantation for CML remains unclear. However, extrapolation from related conditions, such as Philadelphia chromosome positive-acute lymphoblastic leukemia (Ph- Positive ALL), suggests a duration of at least 2 years, up to 5 years.[52,53] Notably, further investigation is warranted to delineate the most efficacious duration TKI maintenance therapy in post-transplant settings for CML patients.
CONCLUSION
While TKI therapy has revolutionized CML treatment, allogeneic SCT remains a potent option for TKI-resistant or intolerant patients and those in advanced phases. Early consideration of transplant in eligible patients promises favorable outcomes, with post-transplant monitoring through real-time quantitative PCR guiding the necessity of TKI maintenance.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Key points
Stem cell transplant is recommended to patients resistant or intolerant to TKI treatment specifically in CML chronic phase. Stem cell transplant is indicated in advanced phase of CML. Stem cell transplant is cost effective in India compare to lifelong TKI treatment. Prior use of TKI does not harm to patients undergoing allogeneic stem cell transplant. Molecular monitoring is required following transplant to track disease progression and response to treatment.
Financial support and sponsorship: Nil.
References
- Chronic myeloid leukemia: A historical perspective. Semin Hematol. 2010;47:302-11.
- [CrossRef] [PubMed] [Google Scholar]
- Transplantation in CML in the TKI era: Who, when, and how? Hematology Am Soc Hematol Educ Program. 2022;2022:114-22.
- [CrossRef] [PubMed] [Google Scholar]
- Evolution of therapies for chronic myelogenous leukemia. Cancer J. 2011;17:465-76.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic myeloid leukemia: Long-term outcome data in the imatinib era. Indian J Hematol Blood Transfus. 2019;35:37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Meeting the needs of CML patients in resource-poor countries. Hematology Am Soc Hematol Educ Program. 2019;2019:433-42.
- [CrossRef] [PubMed] [Google Scholar]
- Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre stop imatinib (STIM) trial. Lancet Oncol. 2010;11:1029-35.
- [CrossRef] [PubMed] [Google Scholar]
- Three decades of transplantation for chronic myeloid leukemia: What have we learned? Blood. 2011;117:755-63.
- [CrossRef] [PubMed] [Google Scholar]
- Complete cytogenetic response and major molecular response as surrogate outcomes for overall survival in first-line treatment of chronic myelogenous leukemia: A case study for technology appraisal on the basis of surrogate outcomes evidence. Value Health. 2013;16:1081-90.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: An analysis from the International randomized study of interferon and STI571 (IRIS) Blood. 2010;116:3758-65.
- [CrossRef] [PubMed] [Google Scholar]
- Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123:1353-60.
- [CrossRef] [PubMed] [Google Scholar]
- European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966-84.
- [CrossRef] [PubMed] [Google Scholar]
- ENESTnd update: Nilotinib (NIL) Vs Imatinib (IM) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) and the impact of early molecular response (EMR) and sokal risk at diagnosis on long-term outcomes. Blood. 2013;122:92-2.
- [CrossRef] [Google Scholar]
- Final 5-year study results of DASISION: The dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333-40.
- [CrossRef] [PubMed] [Google Scholar]
- Report of chronic myeloid leukemia in chronic phase from Tata memorial Hospital, Mumbai, 2002-2008. Indian J Med Paediatr Oncol. 2013;34:164-7.
- [CrossRef] [PubMed] [Google Scholar]
- Therapy in patients with chronic myeloid leukemia outside of clinical trials: Results of the German CML-registry (CML-VI) Blood. 2022;140:947-9.
- [CrossRef] [Google Scholar]
- Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107-12.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: Follow-up of a phase 3 study. Blood. 2014;123:2317-24.
- [CrossRef] [PubMed] [Google Scholar]
- Experience with the use of Nilotinib in Indian Patients. Indian J Hematol Blood Transfus. 2018;34:443-7.
- [CrossRef] [PubMed] [Google Scholar]
- Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Haematologica. 2010;95:224-31.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of 82 chronic myeloid leukemia patients treated with nilotinib or dasatinib after failure of two prior tyrosine kinase inhibitors. Haematologica. 2013;98:399-403.
- [CrossRef] [PubMed] [Google Scholar]
- Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: Final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393-404.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of asciminib treatment in chronic myeloid leukemia patients in real-life clinical practice. Blood Cancer J. 2021;11:16.
- [CrossRef] [PubMed] [Google Scholar]
- Allogeneic transplantation for CML in the TKI era: Striking the right balance. Nat Rev Clin Oncol. 2016;13:79-91.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow aplasia--a rare complication of imatinib therapy in CML patients. Am J Hematol. 2007;82:314-6.
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective analysis of BCR-ABL1 kinase domain mutations in the frontline drug intolerant or resistant chronic myeloid leukemia patients: An Indian experience from a high-end referral laboratory. South Asian J Cancer. 2022;13:132-41.
- [CrossRef] [PubMed] [Google Scholar]
- BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: Recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118:1208-15.
- [CrossRef] [PubMed] [Google Scholar]
- Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: Evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115:1880-5.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of allogeneic hematopoietic cell transplantation in patients with chronic phase CML resistant or intolerant to tyrosine kinase inhibitors. Hematol Oncol Stem Cell Ther. 2022;15:36-43.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of allogeneic hematopoietic cell transplantation in children and young adults with chronic myeloid leukemia: A CIBMTR cohort analysis. Biol Blood Marrow Transplant. 2016;22:1056-64.
- [CrossRef] [PubMed] [Google Scholar]
- Allogeneic stem cell transplantation in chronic myeloid leukemia in era of tyrosine kinase inhibitors-a single centre experience from India. Biol Blood Marrow Transplant. 2019;25:S103.
- [CrossRef] [Google Scholar]
- Cost of hematopoietic stem cell transplantation in India. Mediterr J Hematol Infect Dis. 2014;6:e2014046.
- [CrossRef] [PubMed] [Google Scholar]
- Imatinib mesylate versus allogeneic hematopoietic stem cell transplantation for patients with chronic myelogenous leukemia in the accelerated phase. Blood. 2011;117:3032-40.
- [CrossRef] [PubMed] [Google Scholar]
- HCVAD plus imatinib or dasatinib in lymphoid blastic phase chronic myeloid leukemia. Cancer. 2014;120:373-80.
- [CrossRef] [PubMed] [Google Scholar]
- Allogeneic Stem cell transplantation for blast crisis chronic myeloid leukemia in the era of tyrosine kinase inhibitors: A retrospective study by the EBMT chronic malignancies working party. Biol Blood Marrow Transplant. 2019;25:2008-16.
- [CrossRef] [PubMed] [Google Scholar]
- Imatinib combined with mitoxantrone/etoposide and cytarabine is an effective induction therapy for patients with chronic myeloid leukemia in myeloid blast crisis. Cancer. 2007;109:1543-9.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic myeloid leukemia in blast crisis treated with imatinib 600 mg: Outcome of the patients alive after a 6-year follow-up. Haematologica. 2008;93:1792-6.
- [CrossRef] [PubMed] [Google Scholar]
- Current management of chronic myeloid leukemia myeloid blast phase. Clin Med Insights Oncol. 2022;16:11795549221139357.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of allogeneic stem cell transplant in chronic myeloid leukemia-blast phase: A single-center experience from south India. Indian J Med Paediatr Oncol 2022
- [CrossRef] [Google Scholar]
- Allogeneic stem cell transplantation for blast crisis (BC) chronic myelogenous leukemia (CML) in the tyrosine kinase inhibitors (TKIs) era. Analysis of pre-transplant variables on transplant outcome, On behalf of the Societe Française De Greffe De Moelle Et De Therapie Cellulaire and the French Group of CML. Blood. 2010;116:2266.
- [CrossRef] [Google Scholar]
- Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: A CIBMTR analysis. Bone Marrow Transplant. 2012;47:810-6.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for outcome after allogeneic stem cell transplantation in patients with advanced phase CML. Bone Marrow Transplant. 2021;56:2834-41.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of prior imatinib mesylate on the outcome of hematopoietic cell transplantation for chronic myeloid leukemia. Blood. 2008;112:3500-7.
- [CrossRef] [PubMed] [Google Scholar]
- Novel tyrosine kinase inhibitor therapy before allogeneic stem cell transplantation in patients with chronic myeloid leukemia: No evidence for increased transplant-related toxicity. Cancer. 2007;110:340-4.
- [CrossRef] [PubMed] [Google Scholar]
- Ponatinib both as an effective bridge to allogeneic hematopoietic stem cell transplantation and as posttransplant maintenance therapy in a chronic myeloid leukemia patient with myeloid blast crisis. Hematol Transfus Cell Ther. 2023;45:275-7.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of imatinib mesylate treatment before allogeneic transplantation for chronic myeloid leukemia. Blood. 2007;109:1782-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pre-Transplant use of tyrosine kinase inhibitors increases risk of transplant associated thrombotic microangiopathy-a single centre analysis of incidence, risk factors and outcomes. Blood. 2019;134:1983.
- [CrossRef] [Google Scholar]
- Serial measurement of BCR-ABL transcripts in the peripheral blood after allogeneic stem cell transplantation for chronic myeloid leukemia: An attempt to define patients who may not require further therapy. Blood. 2006;107:4171-6.
- [CrossRef] [PubMed] [Google Scholar]
- Tailored post-transplant maintenance with tyrosine kinase inhibitors for high-risk Philadelphia chromosome-positive leukemia. Biol Blood Marrow Transplant. 2016;22:S313.
- [CrossRef] [Google Scholar]
- Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109:2791-3.
- [CrossRef] [PubMed] [Google Scholar]
- TKI vs relapse after HSCT: Is the jury still out? Blood. 2020;136:1705-6.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of TKIs post-allogeneic hematopoietic cell transplantation in Philadelphia chromosome-positive ALL. Blood. 2020;136:1786-9.
- [CrossRef] [PubMed] [Google Scholar]






