Translate this page into:
Genomic profiling of retinoblastoma using aqueous humor liquid biopsy - A rapid review

*Corresponding author: Purvish Parikh, Department of Clinical Hematology, Sri Ram Cancer Center, Mahatma Gandhi University of Medical Sciences and Technology, Jaipur, Rajasthan, India. purvish1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Agrawal M, Surendran AK, Girish S, Reji RG, Parikh P. Genomic profiling of retinoblastoma using aqueous humor liquid biopsy - A rapid review. Int J Mol Immuno Oncol. 2025;10:11-7. doi: 10.25259/IJMIO_25_2024
Abstract
Background
Retinoblastoma (RB) predominantly affects children under 5 years of age and is the most common primary intraocular tumor in children. The genetic basis of the disease largely involves mutations in the RB1 tumor suppressor gene. Analysis of tumor DNA required invasive procedures but recent studies have identified aqueous humor (AH) as a promising source of tumor-derived cell-free DNA (cfDNA) for non-invasive genetic profiling.
Material and Methods
This rapid review was conducted following Cochrane guidelines and focused on cohort studies providing longitudinal data on RB patients. We searched PubMed, MEDLINE, and the Cochrane Library using relevant keywords on May 24, 2024. Three studies were included after screening for eligibility. Data extraction and risk of bias assessment were performed independently by two reviewers.
Results
Our review found a high detection rate of RB1 pathogenic variants in AH samples, ranging from 89.9% to 100%. The studies demonstrated significant concordance between cfDNA from AH and tumor DNA with variant allele frequencies often exceeding those found in tumor samples. Specific copy number alterations and single nucleotide variants were successfully identified. AH biopsies offered a non-invasive alternative for monitoring treatment response and disease progression, particularly in patients undergoing conservative management.
Conclusion
AH liquid biopsies represent a transformative approach in the genomic profiling of RB, combining high sensitivity and specificity with minimal invasiveness.
Keywords
Aqueous humor
Circulating tumor DNA
Genomics
Liquid biopsy
Retinoblastoma
INTRODUCTION
Retinoblastoma (RB) is a malignant tumor of the retina that usually develops in children before the age of 5 years. Although the incidence of RB is quite low (1/18,000 live births),[1] It is the most common primary intraocular tumor in children and contributes to 3% of all childhood malignancies.[2,3] Nearly half with advanced presentation require enucleation to prevent extraocular spread of the disease.[4] Almost 99% of the RBs can be attributed to the two-hit principle with loss of function of the tumor suppressor gene RB1 being the first hit.[5] This could be due to single nucleotide variants (SNVs), somatic copy number alterations (SCNAs), loss of heterozygosity, or promoter hypermethylation.[6,7] The detection of variations in the RB1 gene provides insights into the development and prognosis of the disease and is important for its early diagnosis as well as for genome-specific, personalized treatment.[8]
Analysis of the tumor DNA has historically only been possible following enucleation or from the tumor biopsy.[9] However, recent studies indicate that aqueous humor (AH) is a rich source of tumor-derived cell-free DNA (cfDNA) which can be used to detect pathogenic variants of the RB1 gene.[10-17] Thus, AH has now been recognized as an enriched liquid biopsy for tumor-related genetic information with a potential clinical utility in the management of children with RB.[11] This rapid review aims to evaluate the current evidence on the effectiveness and reliability of genetic profiling of AH in RB.
METHODOLOGY
Literature search and data extraction
The rapid review was carried out following Cochrane guidelines for rapid reviews.[18] We focused on cohort studies offering longitudinal data on patient populations. This allowed for a thorough evaluation of the diagnostic accuracy and clinical significance of genomic analysis. We searched three databases, “PubMed,” “MEDLINE,” and “Cochrane Library” on May 24, 2024, using the search terms “Genomic analysis,” “Liquid biopsy,” “aqueous humor,” and “retinoblastoma” or their synonyms. We also performed forward and backward citation tracking and identified relevant articles published in the past 6 years based on these search terms. Screening of articles was conducted using the preferred reporting items for systematic reviews and meta-analyses 2020 screening tool. Two authors evaluated titles and abstracts and any articles with ambiguous eligibility were discussed with all authors to reach a consensus. Case studies, case series, editorials, and duplicates were excluded.
The data were extracted using a data extraction table by two authors and accuracy and completeness of the extracted data were assessed by a third. Extracted data included study aim, targeted population, type of intervention, and key findings. The risk of bias and quality of studies were assessed using Cochrane’s ROBINS-I tool and visualized using ROBVIS tool.[19,20] Meta-analysis was not possible as the heterogeneity of the studies did not allow for the same and we conducted a narrative review of principles involved in the included studies.
RESULTS
The screening of studies yielded a total of 19 studies. After removing duplicates case-based studies and editorials, a total of 3 studies were included in the review [Figure 1].[8,12,16] The risk of bias assessment showed a moderate overall risk in the three studies considered for the review and domain D7 showed the lowest risk of bias in all three studies [Figure 2].[19,20]
Patient and sample characteristics
The details of the patient and sample characteristics involved in the studies and the study periods have been described in Table 1.
AH Liquid cfDNA concentration (sequential samples) and sequencing technique
RB1 mutations detected in cfDNA from AH samples in the three studies have been summarized in Table 2.
RB 1 gene mutation detection in cfDNA from AH (pathogenic variant detection)
RB1 pathogenic variants were detected in almost all types of samples except for the ones that had alternative mechanisms (MYCN amplification, 6p gain). The mean detection rate of RB1 pathogenic variants ranged from 89.9% to 100% in different types of samples [i.e., diagnosis (Dx), primary enucleation (PE)]. Samples that could not provide RB1 mutation were either because of an underlying MYCN amplification or insufficient cfDNA in the sample (poor sequencing quality, i.e., low total reads). RB1 pathogenic variant detection means for different samples are given in Table 2. Samples from enucleated eyes were concordant with tumor DNA and those from patients undergoing conservative treatment, and the samples were matched against clinical diagnostic criteria.
RB1 variant allele frequency (VAF)
Targeted sequencing of the samples indicated that more than 90% of the cfDNA is tumor derived with high VAF. Both heterozygote and homozygote VAF for RB1 variants were detected and ranged from 45% to 100% in AH samples. Tumor DNA had relatively low levels of VAF as compared to AH (e.g., one of the cases had 56.5% VAF in AH vs. 6.7% in tumor).
| Study | Number of patients | Bilateral ocular involvement | Enucleation done | Stage of tumor | Follow-up period (months) |
|---|---|---|---|---|---|
| Study 1 (Xu et al.)[12] | 6 | 1 | 2 | cT2b (5), cT2a (1), cT1b (1) | 17.3 (average) |
| Study 2 (Gerrish et al.)[8] | 68 | 19 | 34 (from 68 patients) | Not specified | 12 |
| Study 3 (Schmidt et al.)[16] | 11 | 2 | 11 (all eyes) | cT2b (9), cT1b (1), cT3c (1) | 95 days |
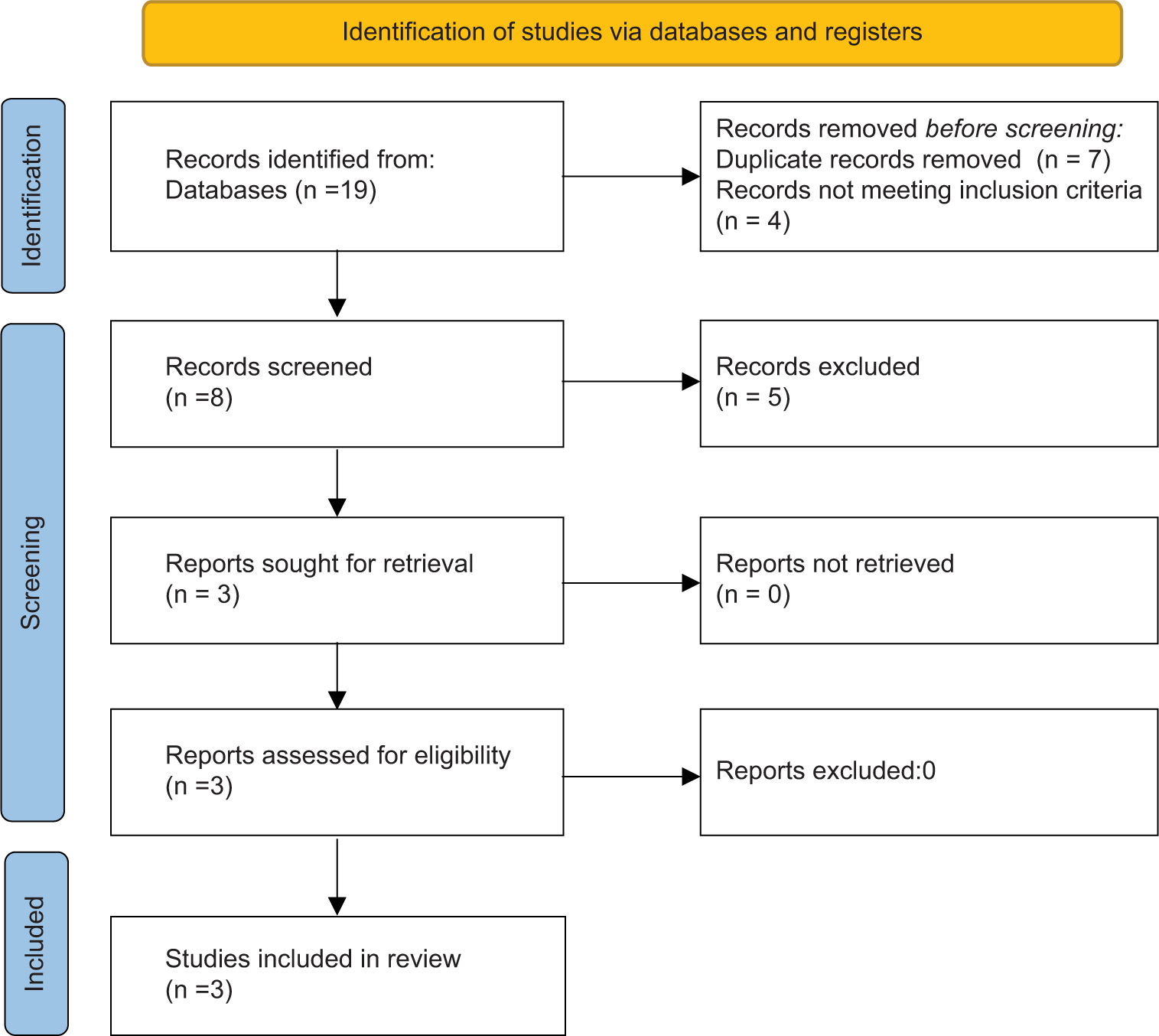
- Preferred reporting items for systematic reviews and meta-analyses flowchart.
| Study | Category | Results | Details |
|---|---|---|---|
| Study 1 (Xu et al.)[12] | RB1 mutation detection in cfDNA from AH | - RB1 variants identified in 5/7 AH samples. - VAF ranged from 66.67% to 100%. |
- 2 samples with no RB1 variants had alternative mechanisms (e.g., MYCN amplification). |
| Study 2 (Gerrish et al.)[8] | RB1 mutation detection in cfDNA from AH | - PE: 91% detection rate (50/55) in primary enumeration samples. - SE : 29% detection rate (4/14) in secondary enucleation samples. - Dx1 +: 75% detection rate (21/28) in anterior chamber tap samples. - Tx : 46% detection rate (25/54) in IViC samples. |
- 5 new somatic RB1 variants were detected - Detection was higher (95%) in Dx ≤2 cases (≤2 chemotherapy cycles). |
| Study 3 (Schmidt et al.)[16] | RB1 mutation detection in cfDNA from AH | - 54.5% of cases had RB1 mutations. - 88.9% concordance between AH and tumor samples. |
- Some variants were detected only in AH or tumor. |
RB: Retinoblastoma, cfDNA: Cell-free DNA, AH: Aqueous humor, VAF: Variant allele frequency, PE: Primary enucleation, SE: Secondary enucleation
RB1 SCNA and SNV
SCNAs and SNVs for RB1 gene were successfully detected in most AH samples. Common RB SCNAs identified included gain of 1q, 2p, 6p, loss of 13q, 16q, etc. Both SCNA and MYCN amplification indicated a poor prognosis in patients. In addition to the detection of SCNAs, high concordance was documented between targeted sequencing and WGS (median 96.2%). Further, some AH samples revealed SCNAs which were not present in their respective tumor samples yielding higher detection rate in AH than tumor samples. However, significance of these SCNAs is yet to be explored.
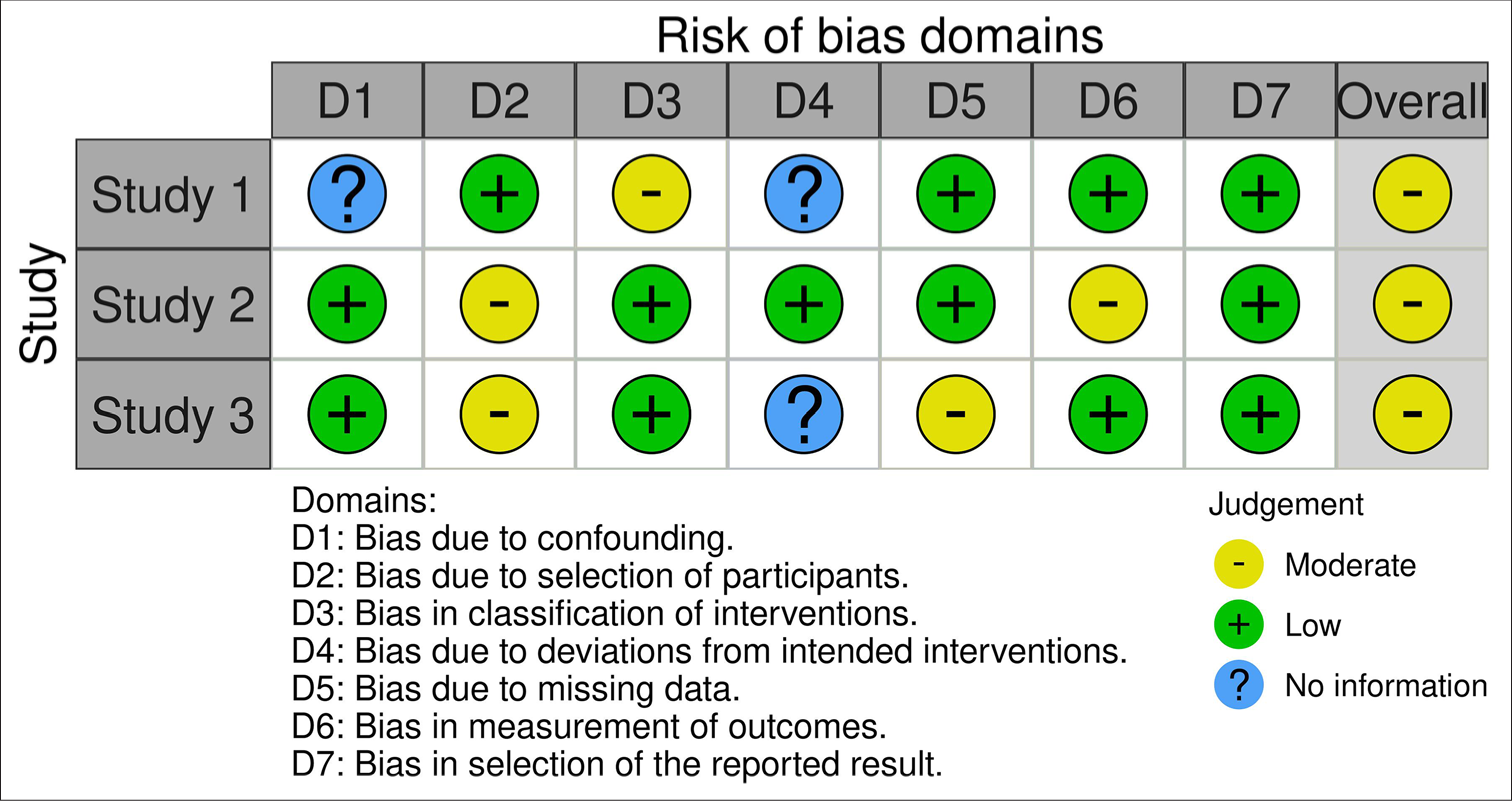
- Risk of bias assessment.
These SCNA detections from AH samples were diagnostic and also altered the treatment outcomes in patients. Similar to SCNA, high number of SNVs were also detected in the studies with 33 RB1 SNVs in study 1. Both SCNA and SNV levels were very low in blood samples indicating poor sensitivity in blood. However, it is essential to note that cfDNA quantity directly affects the detection rates (93% detection rate with more than 250 pg DNA input). Eventually, in patients with conservative treatment, the concentration of cfDNA decreased in response to therapy (in patients with RB1 mutations) leading to decreased rate of detection of SCNA and SNV in these samples.
Concordance between tumor DNA and fraction of cfDNA from tumor-derived fraction
All three studies showed high concordance of RB1 variant detection between AH and tumor samples. Some SCNAs were only found in AH and not in tumor DNA. Concordance for SNVs was reported at approximately 88.9%. Therefore, this indicates an alternative approach for diagnosis complementing enucleation for biopsy of tumor DNA and has the potential to enhance optic survival in RB patients.
RB1 mutation detection in patients undergoing conservative treatment
Sample from different points of duration was taken from patients undergoing intravenous and intra-arterial chemotherapy. These samples included diagnostic, samples after one, two, and some with three cycles of chemotherapy, primary enucleated samples, and secondary enucleated samples. Most of the eyes with RB1 mutation showed excellent response to therapy. Eyes with persistently active intraocular disease were enucleated. Moreover, SCNA analysis from the AH samples and tumor DNA in these eyes were concordant and indicated either MYCN amplification or 6p gain. These patients were followed up for an average of 18–25 months till the completion of their treatment.
DISCUSSION
AH for RB monitoring
AH liquid biopsies were shown to be particularly effective in detecting genomic alterations with a high VAF, often surpassing that found in tumor DNA, as noted in several studies.[8] This review elaborates on the growing potential of genomic profiling using AH liquid biopsies in managing RB. The studies under vision consistently show high detection rates of RB1 gene mutations, with rates ranging from 89.9% to 100% in different types of samples (Dx, PE). The techniques used for the detection of genetic mutations leading to the pathogenesis of RB in the concerned studies identify RB1 pathogenic variants excluding other significant alterations such as MYCN amplification and other specific chromosomal gains. Detection was also not possible in case of inadequate cfDNA in the AH sample. In addition, the ability of AH liquid biopsies to identify specific SCNAs and SNVs that were not present in tumor tissue highlights the superior sensitivity of this method and the significance of this study.
A multitude of screening techniques have been employed for the detection of RB from DNA samples which are used in combination and no single technology carries the efficacy and sensitivity to detect all mutations. Small pathogenic variants are identified through sequencing techniques such as Sanger sequencing and next-generation sequencing (NGS). Array-based methods (array comparative genomic hybridization and single-nucleotide polymorphism arrays), quantitative multiplex polymerase chain reaction, and multiplex ligation-dependent probe amplification (MLPA) can all be used to identify large RB1 deletions or duplications at exonic or chromosomal levels. Sanger sequencing and NGS are used first followed by other techniques like MPLA as germline RB1 mutations are commonly found as small-scale mutations.[21] RB1 copy number variation can be detected by NGS. However, the findings are validated by Sanger sequencing and MLPA.[22,23]
Unlike other cancers, direct biopsy of RB carries a risk of tumor seeding and dissemination beyond the eye.[24] The clinical implications of AH liquid biopsies are profound, offering a non-invasive yet highly effective means of diagnosing, monitoring, and predicting the prognosis of RB.[25] The detection of genomic markers like MYCN amplifications in addition to commonly studied markers like RB1 paves the way to guide tailored treatment strategies including indications for enucleation particularly in MYCN mutations and chromosome 6p gain mutations, leading to improved patient outcomes.[12,26-28] Moreover, AH liquid biopsies may play a pivotal role in predicting treatment response, as genomic alterations in AH cfDNA have been shown to correlate with therapeutic outcomes, particularly in cases undergoing conservative management including intravitreal chemotherapy (IViC) (focal therapy), systemic chemotherapy, focal consolidation with transpupillary thermotherapy, laser photocoagulation and cryotherapy, and radiation treatment with plaque brachytherapy.[12,29]
As demonstrated in studies used for the review as well as various other studies, over time through AH samples allows for dynamic monitoring, the ability to track genomic changes of disease progression, and treatment efficacy. This capability is especially valuable in pediatric oncology, where minimizing invasive procedures is paramount.
Advantages and limitations
The key advantages of AH liquid biopsies include their minimally invasive nature and high concordance with tumor DNA.[11,12,27,28,30-32] This method not only preserves the ocular structure but also provides comprehensive genomic data that can inform clinical decisions, thereby reducing the need for enucleation in many cases.[9] Furthermore, AH liquid biopsies can detect mutations that might be missed in traditional tumor biopsies, particularly in cases with low tumor cell content.[33,34] The AH liquid biopsy has demonstrated established analytical validity through its capacity to accurately and reliably identify RB1 pathogenic mutations and SCNAs, consistently achieving mean concordances exceeding 95% between genomic profiles derived from AH samples and their corresponding tumor tissue.[11,12,27,28]
However, there are limitations to consider. A minimal risk is always expected during the procedure (clear corneal paracentesis and genomic detection).[35] The biopsy needles should only enter the anterior chamber and not make contact with the iris or the lens as it may result in iris scarring or cataracts which may pose setbacks in treatment monitoring. There is also a risk of needle penetrating the vitreous chamber or the tumor which may hypothetically increase the risk of tumor seeding and extraocular dissemination of the disease.[24,36,37] The quantity of cfDNA in AH samples can vary, impacting the sensitivity of mutation detection, given >10 ng of cfDNA is required for detection which is commonly retrieved at the time of diagnosis or primary enucleation.[12,28] Some studies have reported that certain SCNAs detected in AH samples may not be clinically significant, highlighting the need for further research to validate these findings. An additional limitation is that SCNAs cannot be identified at TFxs (tumor fractions) lower than 5%, which compromises the ability to monitor disease in cases where the tumor burden has significantly decreased; eyes that show response to IViC frequently result in this observation. Objective methods for monitoring the response to IViC include fundus photography under general anesthesia and B-scan ultrasonography.[38,39] TFx detection softwares fail to recognize genetic material in cases absence of SCNAs in RB tumors.[28,40-42] Moreover, while AH liquid biopsies have shown promise, standardization of methods across different studies is necessary to ensure consistent and reliable results.
Comparison with other liquid biopsy approaches
Compared to other liquid biopsy methods, such as blood-based cfDNA in analysis, AH liquid biopsies offer distinct advantages in RB management. The identification of SCNAs in the bloodstream is constrained by reduced ctDNA TFx levels and the challenge of establishing a correlation with each eye in 40% of patients who present with bilateral disease.[28] Studies have consistently shown higher detection rates for RB1 mutations and other critical genomic markers in AH samples compared to blood, likely due to the higher concentration of tumor-derived cfDNA in AH. This makes AH a more suitable medium for genomic profiling in RB, offering greater diagnostic accuracy and prognostic value.
Various other biomarkers studied include lactate dehydrogenase,[43] neuron-specific enolase,[44] survivin,[45,46] TGF-β1,[46] and cytokines;[47] other studies also showed elevated protein content in AH as compared to cataracts as controls.[48]
Trefoil family factor peptide 1, a peptide secreted in AH which is expressed ectopically in more advanced subset of RB tumors has emerged as a new biomarker under study in recent years expression of which may indicate more severity and metastases.[49]
Current gaps and future directions
Despite the promising findings, several gaps in the research remain. For instance, the clinical relevance of certain SCNAs detected only in AH samples is not fully understood and warrants further investigation. While the clinical validity of the AH liquid biopsy platform for RB has been confirmed, it is presently authorized solely for research purposes.[27,28,30-32,50] Future research should focus on large-scale, multicenter studies to validate the efficacy of AH liquid biopsies in diverse patient populations. In addition, there is a need to explore the potential of integrating AH liquid biopsy results into routine clinical practice, particularly in combination with other diagnostic tools.
Moreover, advancements in sequencing technologies and bioinformatics could enhance the sensitivity and specificity of AH liquid biopsies, making them an even more powerful tool in the management of RB. Research should also explore the potential of AH liquid biopsies in detecting other ocular tumors, thereby broadening their clinical utility.[51]
CONCLUSION
The systematic review highlights the significant potential of AH liquid biopsies in the genomic profiling of RB. The high detection rates of RB1 mutations and other genomic alterations, combined with the minimally invasive nature of the procedure, suggest that AH liquid biopsies could play a crucial role in the management of RB, ultimately leading to improved patient outcomes and a reduction in the need for invasive procedures.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
Dr. Purvish M. Parikh is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Time to diagnosis of retinoblastoma in Latin America: A systematic review. Pediatr Hematol Oncol. 2019;36:55-72.
- [CrossRef] [PubMed] [Google Scholar]
- Demographics of pediatric orbital lesions: A tertiary eye center experience in Saudi Arabia. J Epidemiol Glob Health. 2019;9:3-10.
- [CrossRef] [PubMed] [Google Scholar]
- Global retinoblastoma presentation and analysis by national income level. JAMA Oncol. 2020;6:685-95.
- [CrossRef] [PubMed] [Google Scholar]
- The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276-80.
- [CrossRef] [PubMed] [Google Scholar]
- Retinoblastoma - Lohmann - major reference works - Wiley Online Library. Available from: https://onlinelibrary.wiley.com/doi/full/10.1038/npg.els.0006053 [Last accessed on 2024 Oct 03]
- [Google Scholar]
- Spectrum of RB1 mutations identified in 403 retinoblastoma patients. J Med Genet. 2014;51:208-14.
- [CrossRef] [PubMed] [Google Scholar]
- RB1 mutation spectrum in a comprehensive nationwide cohort of retinoblastoma patients. J Med Genet. 2014;51:366-74.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic diagnosis of retinoblastoma using aqueous humour-findings from an extended cohort. Cancers (Basel). 2024;16:1565.
- [CrossRef] [PubMed] [Google Scholar]
- Conservative management of retinoblastoma: Challenging orthodoxy without compromising the state of metastatic grace “Alive, with good vision and no comorbidity.”. Prog Retin Eye Res. 2019;73:100764.
- [CrossRef] [PubMed] [Google Scholar]
- Non-invasive diagnosis of retinoblastoma using cell-free DNA from aqueous humour. Br J Ophthalmol. 2019;103:721-4.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous identification of clinically relevant RB1 mutations and copy number alterations in aqueous humor of retinoblastoma eyes. Ophthalmic Genet. 2020;41:526-32.
- [CrossRef] [PubMed] [Google Scholar]
- Establishing the clinical utility of ctDNA analysis for diagnosis, prognosis, and treatment monitoring of retinoblastoma: The aqueous humor liquid biopsy. Cancers (Basel). 2021;13:1282.
- [CrossRef] [PubMed] [Google Scholar]
- Highly sensitive detection method of retinoblastoma genetic predisposition and biomarkers. J Mol Diagn. 2021;23:1714-21.
- [CrossRef] [PubMed] [Google Scholar]
- A typical anterior retinoblastoma: Diagnosis by aqueous humor cell-free DNA analysis. Ophthalmic Genet. 2022;43:862-5.
- [CrossRef] [PubMed] [Google Scholar]
- Inter-eye genomic heterogeneity in bilateral retinoblastoma via aqueous humor liquid biopsy. NPJ Precis Oncol. 2021;5:73.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous copy number alteration and single-nucleotide variation analysis in matched aqueous humor and tumor samples in children with retinoblastoma. Int J Mol Sci. 2023;24:8606.
- [CrossRef] [PubMed] [Google Scholar]
- Chromosome 6p amplification detected in blood cell-free DNA in advanced intraocular retinoblastoma. Ophthalmic Genet. 2022;43:866-70.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: http://methods.cochrane.org/sites/methods.cochrane.org.rapidreviews/files/uploads/cochrane_rr_-_guidance-23mar2020-final.pdf [Last accessed on 2024 Oct 04]
- ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of bias tools - robvis (visualization tool) Available from: https://www.riskofbias.info/welcome/robvis-visualization-tool [Last accessed on 2024 Oct 04]
- [Google Scholar]
- Germline RB1 Mutation in retinoblastoma patients: Detection methods and implication in tumor focality. Transl Vis Sci Technol. 2022;11:30.
- [CrossRef] [PubMed] [Google Scholar]
- Next-generation sequencing-based method shows increased mutation detection sensitivity in an Indian retinoblastoma cohort. Mol Vis. 2016;22:1036-47.
- [Google Scholar]
- Targeted next generation sequencing of RB1 gene for the molecular diagnosis of retinoblastoma. BMC Cancer. 2015;15:320.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor seeding in ocular fine needle aspiration biopsy. Ophthalmology. 1985;92:1763-7.
- [CrossRef] [PubMed] [Google Scholar]
- The Potential of aqueous humor sampling in diagnosis, prognosis, and treatment of retinoblastoma. Invest Ophthalmol Vis Sci. 2024;65:18.
- [CrossRef] [PubMed] [Google Scholar]
- Retinoblastoma with MYCN amplification diagnosed from cell-free DNA in the aqueous humor. Ocul Oncol Pathol. 2024;10:15-24.
- [CrossRef] [PubMed] [Google Scholar]
- Chromosome 6p amplification in aqueous humor cell-free DNA is a prognostic biomarker for retinoblastoma ocular survival. Mol Cancer Res. 2020;18:1166-75.
- [CrossRef] [PubMed] [Google Scholar]
- Genomic cfDNA analysis of aqueous humor in retinoblastoma predicts eye salvage: The surrogate tumor biopsy for retinoblastoma. Mol Cancer Res. 2018;16:1701-12.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances and challenges in the management of retinoblastoma. Indian J Ophthalmol. 2017;65:133-9.
- [CrossRef] [PubMed] [Google Scholar]
- Aqueous humor is superior to blood as a liquid biopsy for retinoblastoma. Ophthalmology. 2020;127:552-4.
- [CrossRef] [PubMed] [Google Scholar]
- Variability in retinoblastoma genome stability is driven by age and not heritability. Genes Chromosomes Cancer. 2020;59:584-90.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal aqueous humor sampling reflects treatment response in retinoblastoma patients. ARVO J. 2020;61:1394.
- [CrossRef] [Google Scholar]
- Liquid biopsies: Applications for cancer diagnosis and monitoring. Genes (Basel). 2021;12:349.
- [CrossRef] [PubMed] [Google Scholar]
- Management of extraocular retinoblastoma: ICMR consensus guidelines. Indian J Pediatr. 2024;91:1157-65.
- [CrossRef] [PubMed] [Google Scholar]
- Aqueous humor as a liquid biopsy for retinoblastoma: Clear corneal paracentesis and genomic analysis. J Vis Exp. 2021;175
- [CrossRef] [Google Scholar]
- Fine needle aspiration biopsy (FNAB) for retinoblastoma. Retina. 2002;22:707-10.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick lecture. Ophthalmology. 1993;100:1677-84.
- [CrossRef] [PubMed] [Google Scholar]
- Intravitreal chemotherapy for vitreous seeding in retinoblastoma: Recent advances and perspectives. Saudi J Ophthalmol. 2013;27:147-50.
- [CrossRef] [PubMed] [Google Scholar]
- Retinoblastoma genetics in India: From research to implementation. Indian J Ophthalmol. 2015;63:219-26.
- [CrossRef] [PubMed] [Google Scholar]
- Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat Commun. 2017;8:1324.
- [CrossRef] [PubMed] [Google Scholar]
- Characterisation of retinoblastomas without RB1 mutations: Genomic, gene expression, and clinical studies. Lancet Oncol. 2013;14:327-34.
- [CrossRef] [PubMed] [Google Scholar]
- Next-generation sequencing of retinoblastoma identifies pathogenic alterations beyond RB1 inactivation that correlate with aggressive histopathologic features. Ophthalmology. 2020;127:804-13.
- [CrossRef] [PubMed] [Google Scholar]
- Lactic dehydrogenase activity of aqueous humour in retinoblastoma. Br J Ophthalmol. 1971;55:130-2.
- [CrossRef] [PubMed] [Google Scholar]
- Electrophoretic determination of aqueous and serum neuron-specific enolase in the diagnosis of retinoblastoma. Zhonghua Yan Ke Za Zhi. 1996;32:219-23.
- [Google Scholar]
- Detection of survivin protein in aqueous humor and serum of retinoblastoma patients and its clinical significance. Clin Biochem. 2010;43:362-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of high levels of survivin and transforming growth factor beta-1 proteins in aqueous humor and serum of retinoblastoma patients. J AAPOS. 2016;20:444.e1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of aqueous humor concentrations of cytokines in retinoblastoma. PLOS One. 2017;12:e0177337.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of aqueous humour proteins in patients with retinoblastoma. Clin Exp Ophthalmol. 2012;40:e8-15.
- [CrossRef] [PubMed] [Google Scholar]
- TFF1 in Aqueous humor-a potential new biomarker for retinoblastoma. Cancers (Basel). 2022;14:677.
- [CrossRef] [PubMed] [Google Scholar]
- Potential of aqueous humor as a surrogate tumor biopsy for retinoblastoma. JAMA Ophthalmol. 2017;135:1221-30.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://main.icmr.nic.in/sites/default/files/guidelines/RB_Guidelines.pdf [Last accessed on 2024 Oct 04]







