Translate this page into:
Outcome in patients of hormone receptor (HR) positive (Her 2) negative metastatic breast cancer treated with palbociclib – A real-world experience

*Corresponding author: Ajay Bapna, Department of Medical Oncology, Bhagwan Mahaveer Cancer Hospital, Jaipur, Rajasthan, India. drabapna@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Bapna A, Samar A, Nag P, Patni S, Patni N. Outcome in patients of hormone receptor (HR) positive (Her 2) negative metastatic breast cancer treated with palbociclib – A real-world experience. Int J Mol Immuno Oncol 2022;1:20-4.
Abstract
Objectives:
We present real-world outcome with the use of palbociclib in patients with HR-positive Her2-negative breast cancer treated at single center in India.
Material and Methods:
We conducted a medical audit of consecutive patients with HR-positive Her2-negative metastatic breast cancer, who were treated with palbociclib at our center between November 2016 and May 2020. Palbociclib was commenced at a dose of 125 mg orally once daily and a schedule of 21 days on therapy followed by 7 days off therapy was followed. Survival analysis included the Kaplan–Meier method using Statistical Package for the Social Sciences software (Version 26). HRs were calculated using Cox proportional hazard regression models and 95% confidence intervals (CIs) for the incidence estimates.
Results:
A total of 67 female patients were commenced on treatment with palbociclib between November 2016 and May 2020. The median age was 55 years (range 29–78 years). A total of 51 (76%) of these patients were postmenopausal and the remaining 16 were premenopausal. Baseline metastatic disease involved one organ/site in 23 (34%), two organs/sites in 32 (48%), three or more in 12 (18%). Bony metastasis alone was seen in 17 (25%) patients, visceral alone in 30 (45%), and the remaining 20 had both bony and visceral metastases. For these 67 patients, palbociclib was commenced as 1st line systemic therapy in 24 (36%) cases. Amongst the remaining 43 cases, it was 2nd line in 21 (31%); 3rd line and beyond in 22 (33%). Median PFS was 16.1 months (95% CI: 9.6–22.8) and median OS was 20.7 months (95% CI: 14.1–27.3). Median PFS for palbociclib use in first line was 18.7 months (95% CI: 4.6–32.9) while in subsequent lines, it was 13.8 months (95% CI: 9.8–17.9; log-rank P = 0.228). Median OS in patients who received palbociclib in first line was 23.2 months (95 % CI 20.1–26.3) and for those why received it in subsequent lines was 16.3 months (95 % CI: 12.5–20.1; P = 0.069). In total population, best response on imaging was CR in 11 (16%) cases (06 in 1st line setting and 05 in subsequent line setting); PR in 33 (49%); SD in 03; and progressive disease in 20. Median PFS with bone only metastasis: 20.9 months (95 % CI: 5.9–36.0), while with visceral metastasis 16.1 months (95% CI: 9.8–22.5; P = 0.537). Median OS with bone only metastasis: 22.7 months (95% CI: 17.8–27.5), while with visceral metastasis, it was 18.5 months (95% CI: 13.6–23.4; P = 0.314).
Conclusion:
Palbociclib is a useful addition in the management of HR +ve Her2 –ve breast cancer patients. Its benefit is confirmed in our real-world setting, both in the first and subsequent lines of therapy and the data are on similar lines as the global real-world data on palbociclib effectiveness.
Keywords
Overall survival
Progression-free survival
India
Patient assistance program
Cost benefit
Quality of life
Hormone receptor positive
her2 negative
INTRODUCTION
Palbociclib has been the first cyclin-dependent kinase 4/6 inhibitor that became available for use in patients with breast cancer.[1] It is now approved for use in all patients with hormone receptor (HR)-positive and human epidermal growth factor receptor 2(Her2)-negative metastatic tumors in combination with aromatase inhibitor (this combination as initial endocrine therapy in MBC patients) or fulvestrant (in patients progressed on prior endocrine therapy). Trials have shown that progression-free survival (PFS) is usually improved by 6–12 months with palbociclib.[2] Our objective was to evaluate the real-world outcome with the use of palbociclib in patients with HR-positive Her2-negative breast cancer treated at our center.
MATERIAL AND METHODS
This is a medical audit of all consecutive patients with breast cancer who were treated with palbociclib at our center between November 2016 and May 2020. Data were collected up to December 31, 2020. Palbociclib was commenced at a dose of 125 mg orally once daily. A schedule of 21 days on therapy followed by 7 days off therapy was followed. Patients were regularly evaluated clinically and with imaging scans as per hospital policy. Prospectively collected data regarding duration of treatment, best response (as per RECIST criteria), additional therapy, adverse events, dose modifications, and status at last follow-up were analyzed. Survival analysis included the Kaplan–Meier method using Statistical Package for the Social Sciences software (Version 26). Hazard ratios (HRs) were calculated using Cox proportional hazard regression models and 95% confidence intervals (CIs) for the incidence estimates.[3] All P-values are two sided.
RESULTS
A total of 67 female patients were commenced on treatment with palbociclib between November 2016 and May 2020. The median age was 55 years (range 29–78 years). A total of 51 (76%) of these patients were postmenopausal and the remaining 16 were premenopausal. ECOG performance status was 1 in 42, was 2 in 24, and 3 in one patient at the start of treatment. The breast primary tumor was on the left side in 31 cases, right side in 35 cases, and one patient had bilateral breast cancer. Baseline metastatic disease involved one organ/site in 23 (34%), two organs/sites in 32 (48%), three in 11, and one patient had involvement of five organs. Bony metastasis alone was seen in 17 (25%) patients, visceral alone in 30 (45%), and the remaining 20 had both bony and visceral metastases. Details of previous cancer directed systemic therapy are shown in [Table 1]. Median duration of hormone therapy was 6 months. Previous hormone therapy included anastrozole (five in adjuvant setting and four in metastatic setting), letrozole (six in adjuvant setting and two in metastatic setting), exemestane (nine in adjuvant setting and five in metastatic setting), tamoxifen (eight in adjuvant setting and one in metastatic disease), and fulvestrant (one in metastatic setting).
| Previous adjuvant systemic therapy | Previous systemic therapy for metastatic disease | ||
|---|---|---|---|
| Hormonal therapy | Chemotherapy | Hormonal therapy | Chemotherapy |
| 28/67 (42%) | 31/67 (46%) | 13/67 (19%) | 14/67 (21%) |
For these 67 patients, palbociclib was commenced as 1st line systemic therapy in 24 (36%) cases. Amongst the remaining 43 cases, it was 2nd line in 21 (31%); 3rd line in 16 (24%); and 4th line in 6 (9%). Median PFS for palbociclib use in first line was 18.7 months (95% CI: 4.6–32.9) while in subsequent lines, it was 13.8 months (95% CI: 9.8–17.9; log-rank P = 0.228). Median OS in patients who received palbociclib in first line was 23.2 months (95 % CI 20.1–26.3) and for those why received it in subsequent lines was 16.3 months (95 % CI: 12.5–20.1; P = 0.069).
Duration of treatment with palbociclib ranged from 1 to 44 months [median 9 months; Table 2]. Of these, 15 patients received it for 4 months or less, 10 due to progressive disease (PD), and 5 due to adverse drug reactions (ADR). In the first-line setting, four patients received it for 4 months or less, two each due to PD and ADR. At last follow-up, palbociclib treatment was ongoing in 25 cases (11/25 were receiving palbociclib in 1st line setting). Best response on imaging was CR in 11 cases (6 in 1st line setting and 5 in subsequent line setting); PR in 33; SD in 03; and PD in 20.
| All cases | 1st line use | Subsequent line use | |
|---|---|---|---|
| n | 67 | 24 (36%) | 43 (64%) |
| Duration of Rx in months– range (Median) | 1–44 (9) months | 1–44 (13) months | 1–42 (8) months |
| Rx for<4 months | 15 (22%) | 04 (17%) | 11 (26%) |
| Rx ongoing at last follow-up | 25 | 11 | 14 |
| Best response (imaging – RECIST criteria) | |||
| CR | 11 | 06 | 05 |
| PR | 33 | 12 | 21 |
| SD | 03 | 02 | 01 |
| PD | 20 | 04 | 16 |
| ADRs | |||
| Total (all neutropenia) | 10 | 05 | 05 |
| Grade 2 | 02 | 02 | 00 |
| Grade 3 | 07 | 03 | 04 |
| Grade 4 | 01 | 00 | 01 |
| Treatment interruption | |||
| (1 month in all cases) | 10 | 04 | 06 |
| Restarting dose | |||
| 75 mg | 02 | 00 | 02 |
| 100 mg | 01 | 01 | 00 |
| 125 mg | 06 | 03 | 03 |
| Not restarted (due to PD) | 01 | 00 | 01 |
While on palbociclib, additional treatment received by the patients included injection zoledronic acid in 41 patients; inj. fulvestrant in 31 cases; tab. letrozole in 35 patients; tab. exemestane in 1 case; inj. leuprolide in 1 patient; and inj. denosumab in 1 case.
The overall survival (OS) and PFS are shown in [Figure 1]. Median PFS was 16.1 months (95% CI: 9.6–22.8) and median OS was 20.7 months (95% CI: 14.1–27.3).
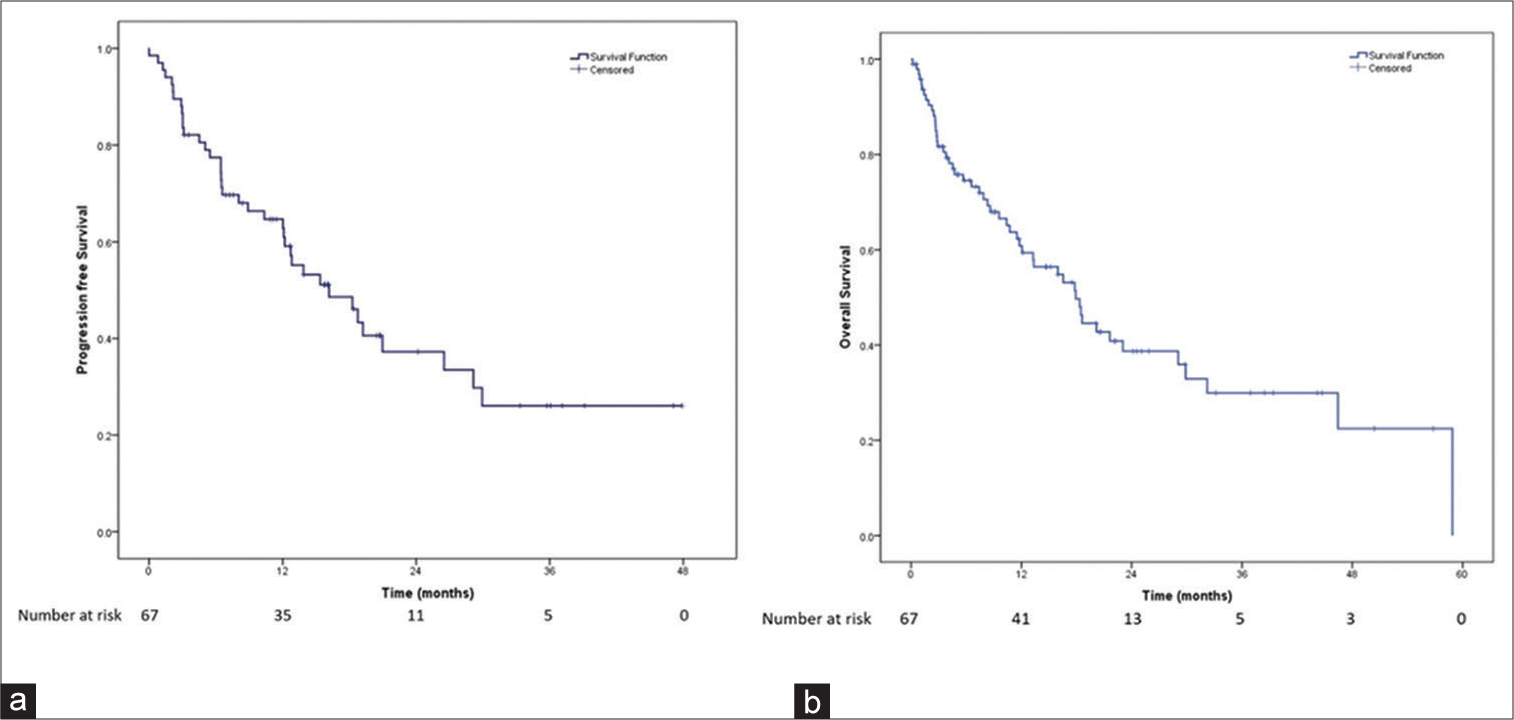
- Survival of breast cancer patients treated with palbociclib. (a) Progression-free survival of the patients receiving palbociclib. (b) Overall survival of the patients receiving palbociclib.
Median PFS with bone only metastasis: 20.9 months (95 % CI: 5.9–36.0), while with visceral metastasis 16.1 months (95% CI: 9.8–22.5; P = 0.537). Median OS with bone only metastasis: 22.7 months (95% CI: 17.8–27.5), while with visceral metastasis, it was 18.5 months (95% CI: 13.6–23.4; P = 0.314).
Median PFS in patients with PS 1 was 18.7 months (95% CI 11.9–25.5) while it was 12.7 months (95% CI 7.1–18.2) in patients with PS 2 (P = 0.324). Median OS in patients with PS 1 was 22.1 months (95% CI 15.7–28.4) while it was 16.4 months (95% CI 9.4–23.4) in patients with PS 2 (P = 0.256).
The only ADR documented was neutropenia. It was self-limiting and recovered in all patients. Its grade is mentioned in the table. As a result of these ADRs, 10 patients required interruption of treatment with palbociclib for 1 month. The drug was reintroduced at lower doses in five patients, as shown in [Table 2]. In one patient, palbociclib was not restarted because she developed PD.
DISCUSSION
The selective CDK 4/6 inhibitor, palbociclib was approved for use in India since October 2016.[4] It is commonly used in combination with letrozole (now aromatase inhibitors) or fulvestrant as first or subsequent lines of therapy in hormone receptor-positive and Her 2-negative metastatic breast cancer. Our medical audit of 67 consecutive patients treated with palbociclib at a single center from November 2016 to May 2020 represents robust real-world data from India. For the whole group, the median PFS was 16.1 months and median OS was 20.7 months. It was used as 1st line therapy amongst 24 of our patients, in whom the median PFS was 18.7 months and median OS was 23.2 months. Interestingly, the best response on imaging was CR in 11 cases, of which five patients had received palbociclib as subsequent line of therapy. Median duration of treatment was 9 months (range 1–44 months). Our median PFS was 16.1 months and median OS was 20.7 months. This translates into better quality of life and postponement of need for chemotherapy.[5]
The PFS and toxicity analysis results are consistent with other reports of routine clinical practice, that is, real-world evidence from India and other countries and the global clinical trials.[6-17] The observed OS is lower than reported in real-world studies and clinical trials. Our analysis suggests that patients who were less heavily pre-treated had better outcomes. Our population was mainly in second or later lines (64%), probably contributing to the low OS.
Palbociclib was tolerated well in our patient population with neutropenia as the major adverse event. About 15% of patients required drug interruption, 7.5% dose reduction, but no patient required drug discontinuation. The safety profile of palbociclib was consistent with that seen in clinical practice and trials in India and other countries.[6,10-12,15,16]
The major limitation of our study is its retrospective nature as that introduces a potential bias in patient selection, also it being a single-institutional single-arm study with a small sample size.
Use of palbociclib in second or later lines is associated with shorter duration of use, and hence, lower cost compared to use in first line, if the patients are not covered by health insurance and have to spend money for treatment. However, the global guidelines recommend use of palbociclib and other CDK 4/6 inhibitors as initial line of therapy for optimum benefit. To aid this, organizations like Pfizer have introduced patient assistance programs to lessen the economic impact for patients receiving these drugs in approved indications.
There is a further need to evaluate the quality of life of patients being treated with palbociclib in clinical practice, impact of biomarkers on the patient prognosis, and the mechanisms of resistance to CDK 4/6 inhibitors, requiring further studies in Indian population.
CONCLUSION
Palbociclib has a place in the management of HR +ve/ Her2 – ve breast cancer patients.[18] Subsets of patients that can potentially benefit have been confirmed in our real-world setting, both in the first and subsequent lines of therapy.[19,20] Adverse events seen in our patients are similar to those reported in literature and we did not see any new safety red flags.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425-39.
- [CrossRef] [Google Scholar]
- Real-world single centre experience with Palbociclib as first line treatment in Indian patients with metastatic breast cancer. J Clin Oncol. 2018;36:e13030. Available from: https://www.ascopubs.org
- [CrossRef] [Google Scholar]
- Advanced lung cancer and molecular testing in the middle of COVID-19 second wave in the SAARC region. Int J Mol Immuno Oncol. 2021;6:61-5.
- [CrossRef] [Google Scholar]
- Palbociclib and fulvestrant in breast cancer. N Engl J Med. 2019;380:796.
- [CrossRef] [Google Scholar]
- An evaluation of palbociclib as a breast cancer treatment option: A current update. Expert Opin Pharmacother. 2021;22:281-90.
- [CrossRef] [PubMed] [Google Scholar]
- Real-world safety of palbociclib in breast cancer patients in the United States: A new user cohort study. BMC Cancer. 2021;21:97.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/ HER2-metastatic breast cancer in US real-world clinical practice. Breast Cancer Res. 2021;23:37.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment patterns and clinical outcomes among patients receiving palbociclib in combination with an aromatase inhibitor or fulvestrant for HR+/HER2-negative advanced/metastatic breast cancer in real-world settings in the US: Results from the IRIS study. Breast. 2019;43:22-7.
- [CrossRef] [PubMed] [Google Scholar]
- Real World Treatment Patterns and Clinical Outcomes Associated with Palbociclib Combination Therapy across Five European Countries: Results from the IRIS Study, Presented at the ESMO Virtual Congress, 19-21 September 2020. 2020:269.
- [CrossRef] [Google Scholar]
- Palbociclib in hormone positive metastatic breast cancer: A real world multicenter Indian experience. J Clin Oncol. 2020;38:e13057.
- [CrossRef] [Google Scholar]
- Real-World evidence of palbociclib use in metastatic hormone positive HER negative metastatic breast cancer in Indian population. Eur J Cancer. 2020;138(Suppl 1):S18-2.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of palbociclib and ribociclib in patients with estrogen and/or progesterone receptor positive, HER2 receptor negative metastatic breast cancer in routine clinical practice. PLoS One. 2021;16:e0253722.
- [CrossRef] [PubMed] [Google Scholar]
- Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174:719-29.
- [CrossRef] [PubMed] [Google Scholar]
- Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926-36.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Updated analysis with up to 5 years of follow-up. Oncologist. 2021;26:e749-55.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HER2-advanced breast cancer. J Natl Cancer Inst. 2019;111:419-30.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol. 2018;29:669-80.
- [CrossRef] [PubMed] [Google Scholar]
- Afro Middle East Asian symposium on cancer cooperation. South Asian J Cancer. 2014;3:128-31.
- [CrossRef] [PubMed] [Google Scholar]
- ICON 2013: Practical consensus recommendations for hormone receptor-positive Her2-negative advanced or metastatic breastcancer. Indian J Cancer. 2014;51:73-9.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: Patient-reported outcomes from the PALOMA-3 trial. Ann Oncol. 2016;27:1047-54.
- [CrossRef] [PubMed] [Google Scholar]






