Translate this page into:
Assessment of mismatch repair protein expression by immunohistochemistry in endometrial carcinomas with clinicopathological correlation: A study from Indian Tertiary Cancer Care Centre

*Corresponding author: Anila Sharma, Department of Pathology, Rajiv Gandhi Cancer Institute and Research Centre, Rohini, New Delhi, India. dranila_sharma@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Sharma A, Kamboj M, Panaych A, Gupta G, Pasricha S, Jain V, Mehta A. Assessment of mismatch repair protein expression by immunohistochemistry in endometrial carcinomas with clinicopathological correlation: A study from Indian Tertiary Cancer Care Centre. Int J Mol Immuno Oncol 2020;5(3):101-7.
Abstract
Objectives:
Endometrial carcinomas (EC) are known to be histologically and biologically heterogeneous, and their recent molecular characterization has highlighted their etiologic heterogeneity. The aim of the present study was to analyze mutations in mismatch repair (MMR) proteins in ECs by immunohistochemistry (IHC), and correlates the data with their pathological parameters.
Material and Methods:
The expression of MMR proteins was analyzed using IHC in VENTANA BENCHMARK XT system, on formalin-fixed paraffin-embedded tumor tissue. The study population included 102 newly diagnosed cases of ECs over a duration of 2 years.
Results:
On histopathologic subtyping, 85.1% of cases were of Type 1 EC, 9.8% were Type 2 EC, and 4.9% were malignant mixed Mullerian tumors. On IHC for MMR protein expression, 22 of 102 cases (21.6%) showed loss of one or more protein, and mean age of patients with deficient MMR (dMMR) was 59.6 years. All of these dMMR cases were of endometrioid subtype, forming 25.3% of EEC. The combined loss of MLH1 and PMS2 was the most common abnormality detected (50% of dMMR). On pathological correlation, 54.5% of dMMR cases were found to be of higher grade (grade 2/3; P = 0.002) and 68.2% were higher stage tumors (T1b and above; P < 0.0001). The lymph-vascular invasion was seen in 50% of dMMR cases (4 of 8 cases).
Conclusion:
Detecting MMR protein loss in ECs by IHC is an efficient, relatively simple, and economical method. It needs to be routinely performed in all cases of ECs. Studies are still underway to utilize it as a therapeutic modality using immunotherapy.
Keywords
Endometrial carcinoma
Immunohistochemistry
Lynch syndrome
Microsatellite instability
Mismatch repair
INTRODUCTION
Endometrial cancer is the 4th most commonly diagnosed gynecologic malignancy in the world, and 11th most common in India.[1] Most of the uterine cancers are endometrial carcinomas (EC), with known histologic and biological heterogeneity. The recent molecular classification of ECs has further emphasized their etiologic heterogeneity.[2]
Traditionally ECs are subtyped as Bokhman Type 1 and 2 based on histological features showing different metabolic and endocrine signals.[3] Type 1 EC is more common (~70–80%), with prototype endometrioid carcinoma, which is low grade, hormone- driven tumors, usually with a favorable prognosis; whereas Type 2 tumors (~20–30%) with non-endometrioid histology, which includes serous, clear cell, and mixed carcinomas, have high-grade features, are hormone receptor-negative, with a higher risk of metastasis and poor prognosis. However, this morphologic subtyping and grading, especially in the high grade tumors, has inter-observer variability and thus, is not formally incorporated for risk stratification.[2,3] Over 75% of patients with EC present with early-stage disease (Stage I or II) with favorable outcome (5-year overall survival 75–90%). Women with the advanced and recurrent tumors show poor clinical outcomes with conventional chemotherapy.[3]
Risk factors for EC include hyperestrinism, obesity, nulliparity, and hereditary factors.[2] Among hereditary cancer syndromes associated with ECs, lynch syndrome (LS) is the most prevalent seen as a result of inherited mutations in mismatch repair (MMR) genes.[2,4] Although the vast majority of ECs with deficient MMR (dMMR) proteins are sporadic, 3–5% of cases develop as a result of inherited mutations in DNA MMR genes.[2] Immunohistochemistry (IHC) for MMR proteins is also emerging as a tool for screening for LS.[5]
We analyzed loss of expression of MMR proteins by IHC in newly diagnosed cases of EC and correlated them with the histological type and pathological parameters.
MATERIAL AND METHODS
An observational study was conducted including 102 cases of EC diagnosed from January 2014 to January 2016 (duration of 2 years), at Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India.
Collection of tumor specimen and data
The demographic details of patients and histopathological parameters of EC were documented, including the subtype, grade, and stage of the tumor. IHC for MMR proteins was performed on formalin-fixed paraffin-embedded (FFPE) blocks of tumor tissue from resected specimens in all 102 cases.
IHC
IHC analysis of MLH1, MSH2, MSH6, and PMS2 was performed on FFPE tissue sections, using specific mouse monoclonal antibodies on VENTANA BENCHMARK XT system. Paraffin sections (4 μm thick) affixed to adhesive slides were air-dried overnight at room temperature, and after de-paraffinization, sections were transferred to automated immunostainer (VENTANA BENCHMARK XT). IHC analysis was performed using the primary antibodies MLH1 (p16 E6H4, Ventana), PMS2 (EPR 3947, Cell Marque), MSH2 (CG219-1129, Cell Marque), and MSH6 (SP93, Cell Marque). Binding of the primary antibody was detected by OV H2O2 and OV DAB, followed by counterstaining with hematoxylin.
Analysis of immunoreactivity
The analysis of immunoreactivity in the tumor samples was analyzed by two independent pathologists, AS and GG. Only the complete absence of nuclear staining in all tumor cells was accepted as negative expression/loss of an MMR protein. Adjacent normal endometrium, stromal cells, and lymphocytes were taken as an internal positive control. The expression of MMR proteins was correlated with various pathological parameters.
RESULTS
A total of 102 cases of endometrial carcinoma (EC) collected over 2 years were analyzed. The histopathologic features with tumor subtype, grade, stage, and the MMR status using IHC are tabulated in Table 1.
| Parameters | Total | Type 1 endometrial carcinoma | Type 2 endometrial carcinoma | Others |
|---|---|---|---|---|
| Age (mean) | 34–91 years (59.6 years) | 34–85 years (58 years) | 60–91 years (69.7 years) | 50–75 years (66.8 years) |
| Histopathologic subtype | 86-Endometrioid | 7-Serous | 5-Malignant mixed Mullerian tumor | |
| 1-Mucinous | 2-Mixed | |||
| 1-Clear cell | ||||
| Tumor grade of Type 1 EC | Grade 1-55 (63.2%) | Not applicable | Not applicable | |
| Grade 2-21 (24.1%) | ||||
| Grade 3-11 (12.6%) | ||||
| Stage -T1a | 50 (49%) | 46 | 1 | 3 |
| T1b | 33 (31.4%) | 29 | 3 | 1 |
| T2 | 9 (8.8%) | 7 | 2 | 0 |
| T3a | 9 (8.8%) | 5 | 4 | 0 |
| T3b | 1 (1.0%) | 0 | 0 | 1 |
| Mismatch repair protein status | ||||
| Intact | 80 (78.4%) | 65 (74.7%) | 10 (all cases) | 05 (all cases) |
| One or more loss | 22 (21.6%) | 22 (25.3%) | 0 | 0 |
| Total | 102 | 87 (85.1%) | 10 (9.8%) | 5 (4.9%) |
Patient age ranged from 34 to 91 years, with a mean of 59.6 years. On histopathologic subtyping, 85.1% (87/102) cases were of Type 1 EC (86 endometrioid carcinoma, and 1 mucinous carcinoma), 9.8% (10/102) cases were Type 2 EC (7 serous, 1 clear cell, and 2 mixed carcinomas), and 4.9% (5/102) cases were malignant mixed Mullerian tumors (MMMT). Type 1 EC had a younger mean age of 58 years, as compared to 69.7 years for Type 2 EC and 66.8 years for MMMT. Among 86 EECs, 55, 21, and 11 belonged to the International Federation of Gynecology and Obstetrics (FIGO) Grades 1, 2, and 3, respectively [Figure 1]. According to the FIGO, staging classification majority of tumors were in stage T1a, comprising 50 of 102 cases, followed by T1b with 33 cases, T2 and T3a, each with 9 cases and T3b with 1 case.
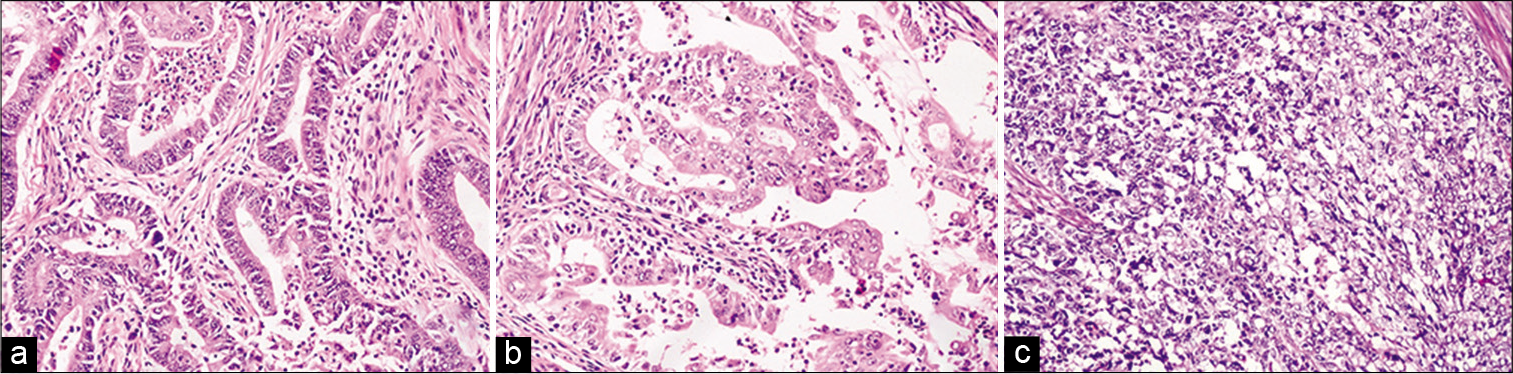
- (a-c) Histomorphology of endometrioid adenocarcinoma Grades 1, 2, and 3, respectively. (H&E, ×200).
Using IHC for MMR protein expression, 22 of 102 cases of EC (21.6%) showed lost expression of one or more proteins, while 80 (78.43%) cases had an intact expression of all MMR proteins. All of dMMR cases belonged to Type 1 EC, while none of the Type 2 EC or MMT showed a loss of MMR protein expression. Among endometrioid EC (EEC), 25.3% (22/87) cases had dMMR protein expression [Figure 2].
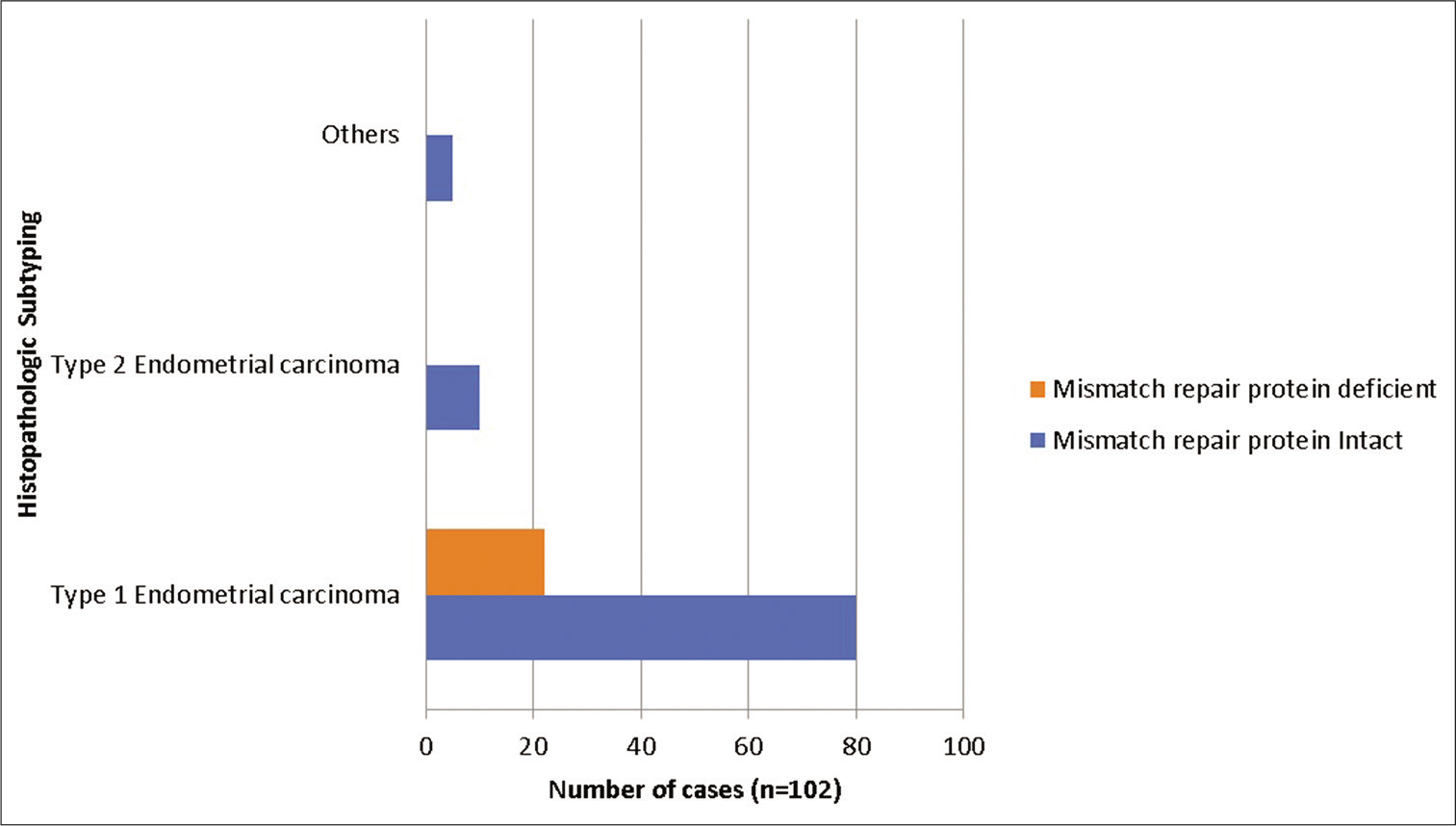
- Mismatch repair protein status using immunohistochemistry in endometrial carcinomas.
In the present study, the combined loss of MLH1 and PMS2 was the most common abnormality detected, seen in 50% (11/22) of dMMR cases. Isolated loss of PMS2 was seen in 4 cases (18.2% of dMMR), among which two were of Grade 1, while one was of Grade 3. The combined loss of MSH2 and MSH6 was seen in 3 cases (13.6% of dMMR), and all had Grade 2 tumors. Isolated loss of MSH6 was seen in 3 cases (13/6% of dMMR), which included 2 cases of Grade 1 tumors and one case of Grade 2 tumor. Loss of all four MMR proteins was seen in a single case (4.5% of dMMR), which was of morphological Grade 3 [Figure 3 and Table 2].
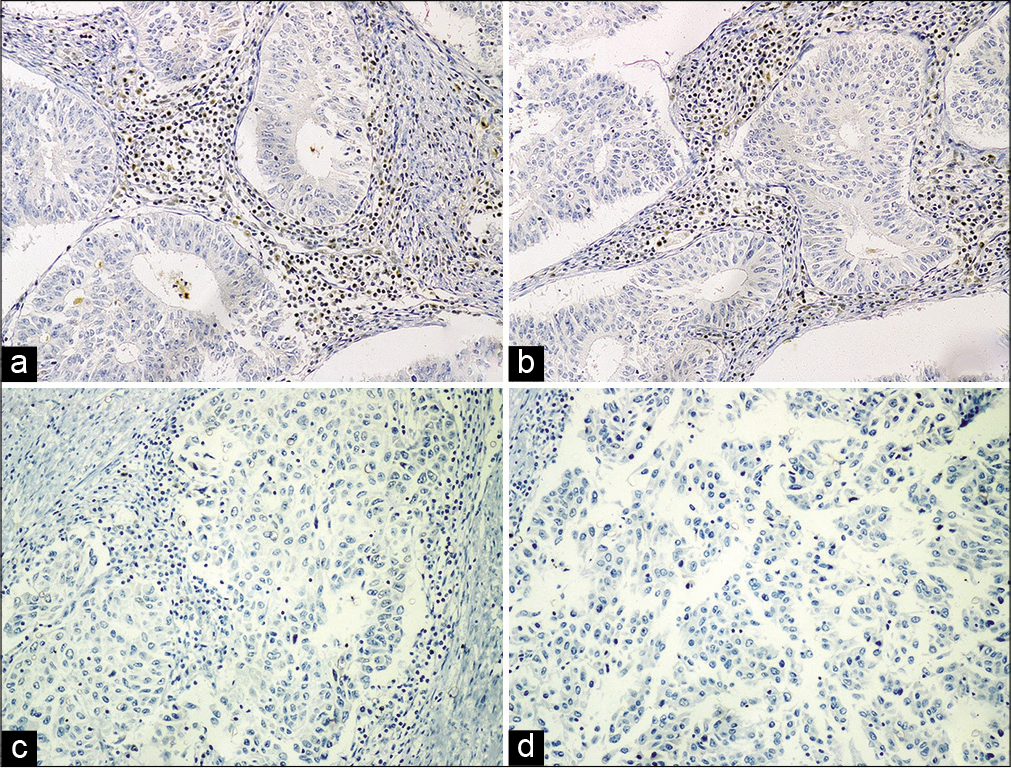
- Immunohistochemical analysis of MMR proteins in endometrial carcinomas. (a) Loss of expression of MLH 1 in Grade 1 EEC; (b) loss of expression of MSH 2 in Grade 2 EEC; (c) loss of expression of MSH 6 in Grade 3 EEC; (d) loss of expression of PMS 2 in Grade 3 EEC. Positive internal control of lymphocytes with intact nuclear expression is seen in all figures.
| Parameters | Loss of mismatch repair protein (n=22) (all were Endometrioid carcinoma) | ||||||
|---|---|---|---|---|---|---|---|
| Total cases with deficient mismatch repair protein | Loss of MLH1 and PMS2 | Loss of MSH2 and MSH6 | Loss of PMS2 | Loss of MSH6 | Loss of All proteins | ||
| Number of cases | 22 | 11 (10.78%) | 3 (13.6%) | 4 (18.2%) | 3 (13.6%) | 1 (4.5%) | |
| Mean age (years) | 59.6 | 60.5 | 46.7 | 56 | 57 | 53 | |
| Grade-1 | 10 | 6 | 0 | 2 | 2 | 0 | Low versus High grade, P=0.002 |
| 2 | 7 | 2 | 3 | 1 | 1 | 0 | |
| 3 | 5 | 3 | 0 | 1 | 0 | 1 | |
| Stage-T1a | 8 | 1 | 1 | 3 | 3 | 0 | |
| T1b | 9 | 6 | 1 | 1 | 0 | 1 | |
| T2 | 5 | 4 | 1 | 0 | 0 | 0 | |
| T3a | 0 | 0 | 0 | 0 | 0 | 0 | |
| T3b | 0 | 0 | 0 | 0 | 0 | 0 | |
| Myometrial invasion | |||||||
| More than half | 15 | 10 | 3 | 1 | 0 | 1 | |
| Less than half | 7 | 1 | 0 | 3 | 3 | 0 | |
Among the 22 cases of dMMR protein expression, 12 (54.5%) cases had a higher tumor grade (Grades 2 and 3), and 10 cases (45.5%) had Grade 1 tumor, which was statistically significant
(P = 0.002). It was observed that 14 out of 22 (68.18%) dMMR cases were in higher tumor stage (T1b and above), which was statistically significant (P < 0.0001). High stage tumors with dMMR comprised 28.3% (15/53) cases [Table 2].
Lymph-vascular invasion (LVI) was seen in eight cases, of which 4 had dMMR - 2 with combined loss of MLH1/PMS2, 1 case with isolated loss of MSH2, and 1 case with combined loss of MSH2/ MSH6 [Table 2].
Follow-up was not available in most of the patients. Three patients with dMMR had succumbed to illness (2 with loss of MLH1/PMS2, and 1 with isolated loss of PMS2).
DISCUSSION
The role of microsatellite instability (MSI) and carcinogenesis was first elucidated in hereditary non-polyposis colorectal cancer or LS, following which its role has been demonstrated in many other cancers.[6] The accumulation of micro-deletions and micro-insertions in microsatellite regions, which are located in coding and regulatory regions of suppressor genes and proto-oncogenes, leads to change in the expression of these genes and malignant transformation.[4] MSI arises from genetic (inherited) or sporadic/epigenetic defects in the post- replicative DNA MMR system, which results in a greatly increased rate of strand slippage mutations.[2,4]
LS shows the presence of germline mutations in one or more of the MMR genes - MSH2, MLH1, MSH6, and PMS2.[7,8] The majority of MSI in colorectal carcinoma (CRC) and EC is caused by defects in MMR genes MLH1, MSH2, and MSH6.[6] Among LS associated cancers, EC is the second most common cancer after CRC, and the most frequent extra-colonic cancer, implying that MSI may have a role in endometrial carcinogenesis and prognosis.[9-11] LS-associated genetic mutations have been found in 3–5% of ECs,[2] and lifetime risk of EC for women with LS is 54%.[12] NRG Oncology and Gynecologic Oncology Group (NRGO/GOG) 210 trial estimated LS association in ECs to 3.89%.[11] Tumors with MSI-H or IHC loss of expression of MMR proteins in the absence of MLH1 gene methylation are suggestive of LS.[12]
The prevalence of MSI in ECs with LS has been reported in 75% cases, and 20–43% in sporadic cases.[6] Incidence of MSI in Type 1 EC has been documented to range from 20 to 40% in varying studies[11,13] which is twice that seen in CRC[2]and 0–11% in Type 2 EC.[14,15]
The assessment of MMR deficiency can be done either by DNA extraction from normal and tumor tissue for MSI assay, amplification of selected microsatellites by PCR, and analysis of fragment size by gel electrophoresis or IHC.[6] IHC detection is based on the demonstration of complete loss of protein product of MSI related genes. The most effective method of assessment is to test for MSI genetic mutations; however, IHC is a cost effective and simpler technique, and it also identifies the mutated gene.
The importance of MSI status has been highlighted in the recent molecular classification of EC, with four distinct molecular subgroups. The Cancer Genome Atlas (TCGA) project classified ECs into four groups – POLE ultramutated (POLE EDM), MSI hypermutated (MMR deficient, MSI), copy-number (CN) high (p53 mutation), and CN low (NSMP – no specific molecular profile). It has been observed that patients belonging to the POLE mutation and MSI subgroups showed much better survival outcomes in comparison to the p53 mutant group and the NSMP group.[3]
In TCGA, MSI was determined by a panel of four mononucleotides and three dinucleotide repeat loci. Tumor DNA was classified as microsatellite-stable (MSS), low-level MSI (MSI-L), or high-level MSI (MSI-H) based on alteration in none of the markers, 1-2 markers (<40%), and >3 markers (>40%), respectively.[3]
Histomorphologic surrogates for abnormal MMR IHC/MSI (MMR morphology) in ECs include tumor infiltrating lymphocytes, peritumoral lymphocytes, undifferentiated tumor subtype, lower uterine segment origin, and/or concurrent ovarian cancer. However, they have not proven to be equivalent to molecular confirmation.[3,16] Very scant literature regarding MMR morphology in EC is available, with no specific guidelines of assessment and diagnostic reproducibility.[17]
Increasing evidence for LS screening in newly diagnosed EC using tumor MSI and/or MMR IHC is emerging.[8,18,19] The Amsterdam II and revised Bethesda criteria used to identify patients of CRC through screening for LS, using criteria such as age, family, and personal history, remain insufficient as almost 25% of LS patients do not meet the standard screening criteria.[5]
There has been a debate about whether to use MMR as selective screening in patients fulfilling the criteria along with MMR morphology, or as universal screening. A retrospective study conducted in patients of EC who did not meet the criteria for selective screening (patients of >50 years and lacking MMR morphology), had 66% cases lacking criteria for screening, and 97.8% of these had intact MMR. Among the dMMR cases, one had loss of MSH6 (0.5%) which was high grade serous carcinoma, and three had loss of MLH1/PMS2 (7%), all due to MLH1 promoter hypermethylation, which being an epigenetic event rules out LS. Based on these findings, the study suggested universal MSH6 and selective MLH1, PMS2, and MSH2 testing by IHC.[17]
Challenges in implementing universal screening include financial limitations. According to the current NCCN guidelines (2019), screening in ECs has not shown proven benefit with LS; however, screening through endometrial biopsy every 1–2 years may be considered.[20]
A large cohort of ECs recommended combined MMR protein IHC, MLH1 methylation test, and MSI test to screen for LS in all patients of EEC, regardless of age.[11] Other studies have suggested testing all patients of EC with IHC, regardless of age and family history.[21] The overall sensitivity of detecting germline LS mutations by IHC for MMR protein is 94%, as compared to detection by MSI testing with an overall sensitivity of 83%.[12]
Joehlin-Price et al.,[22] in his study, had dMMR in 22.4% cases, comparable to 21.6% observed in our study. The mean age of patients with dMMR protein (by IHC) in our data was 59.6 years, comparable to other similar reports.[5,22] In our study, 90.9% of cases with dMMR were >50 years of age; a similar study had 62% of dMMR cases ≥50 years of age.[8] Among cases with dMMR proteins, those exhibiting loss of MLH1 and PMS2 were older (mean 60.5 years) than those with loss of MSH2 and/or MSH6 (mean 46.7 years), similar to finding by Joehlin-Price et al.[22]
All the 22 cases with dMMR in our data were EEC. However, dMMR in other subtypes has also been reported by some authors; Long et al.[5] reported dMMR in 82.9% EECs, 9.7% serous, and 7.3% clear cell carcinomas, and Catasus et al.[14] reported 33.3% of EECs and 11% of serious carcinomas with MSI.
MMR defects have been shown to correlate with negative prognostic factors such as higher-grade tumor, presence of LVSI, and higher stage in NRG/GOG study, which analyzed combined MSI, MLH1 methylation and IHC for MMR in 1024 ECs. However, the association of higher grade with MSI phenotype is significant only when EEC are considered.[2] Statistically significant correlation of dMMR with higher grade (P = 0.002) was seen in our study, similar to seen by Long et al.[5] (P = 0.003). Several other studies have shown similar results – NRG/GOG trial[2] had 66% of dMMR cases of grade 2/3, and another study[23] reported MSI in 35% of Grade 3 tumors. A study[6] conducted on MSI in Grade 3 ECs using IHC, 27.4% were diagnosed as MSI tumors. Among these, 56% were of Type 1 and 44% type 2EC; and 48% of these MSI tumors were in advanced stage (FIGO III/IV).
Our data also had 68.18% of dMMR with a higher tumor stage (≥T1b), and half of the cases with LVI had dMMR. In NRGO/GOG trial, LVI was seen in 32.77% of EC with dMMR, as compared to 17.13% with normal MMR, and no association was seen with the depth of invasion.[2] Another study has reported significant deeper myometrial invasion (P = 0.008) and positive lymph nodes metastasis (P = 0.002) in dMMR cases.[5] Within the stage subgroup, better outcomes are expected in MMR deficient tumors as they elicit an anti-tumor immune response.[2] Similar to our study, the combined loss of MLH1/PMS2 was the most common abnormality reported by Joehlin-Price et al.,[22] whereas Long et al.[5] reported the most common abnormality as loss MSH2/MSH6 (51.2%).
High concordance rates between MMR IHC and MSI assay methods have been reported, ranging from 92% to 100%.[24,25] A study on new cases of EC reported MSI-H in 25.1%, dMMR in 27.4%, and MLH1 methylation in 93.5%; with a concordance rate of 96.9%.[12] Possible reasons for discordant results include the presence of abnormal MMR genes not covered in antibody panels, present but non-functioning MMR genes, antigen degradation, tumor heterogeneity, or inability of MSI testing to detect MSH6 mutations.[25]
The proportion of MSH6 mutations is higher in EC than CRC with LS, and MSH6 carriers can exhibit MSI-low or MSS phenotype. In such a scenario MMR IHC is the preferred method of testing that might otherwise be missed.[25]
MSI is an independent predictor of a favorable outcome in CRC, but its association in ECs is still controversial.[2,6] Some studies suggest an association of MSI with improved outcomes in EC, while others have indicated worse or no prognostic significance.[6,16] The different methodologies used to assess MMR abnormalities and the histological variants of EC may account for the discordant findings.[16] A better survival has been observed in MMR mutated tumors than MMR epigenetic defects; however, on multivariate analysis, no association with the outcome has been seen. Adjuvant therapy shows a four-fold advantage in MMR mutated tumors as compared to normal tumors.[2]
Benefits of IHC for MMR include less expensive and simple technique and the presence of internal control for reporting.[12] The loss of IHC staining of MMR proteins is related to the corresponding MMR gene mutation and thus is useful in guiding the genetic mutation testing for patients and families at risk for LS, especially if EC is the first occurrence of cancer in the LS family.[26,27]
Drawbacks of reporting MMR protein by IHC include inter-observer disagreement and ambiguous heterogeneous results, which can be affected by a number of biological and technical factors. Timely fixation to reduce cold ischemic time, adequate fixation in 10% neutral buffered formalin, appropriate thickness of sections, and if required, repeat test on a different section helps to overcome these factors. Another disadvantage includes false-positive IHC stain seen in missense MLH1 and MSH6 mutations, leading to truncated protein with retained antigenicity.[5]
Our study was limited by the non-availability of clinical follow-up and being a retrospective study, the further genetic study could not be obtained.
CONCLUSION
Conventional IHC detection of MMR protein expression is a cheap, efficient, and simple tool to detect MSI phenotype. New clinical treatment options for patients with dMMR or MSI-H in EC, considering it an immunogenic tumor, are being investigated, including the use of checkpoint inhibitors.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic significance of mismatch repair defects in endometrial cancer: An NRG oncology/ gynecologic oncology group study. J Clin Oncol. 2016;34:3062-68.
- [CrossRef] [PubMed] [Google Scholar]
- New classification of endometrial cancers: The development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract. 2016;3:14.
- [CrossRef] [PubMed] [Google Scholar]
- The study of mismatch repair in endometrial cancer patients with a family history of cancer. Exp Oncol. 2015;37:272-6.
- [CrossRef] [Google Scholar]
- Role of endometrial cancer abnormal MMR protein in screening lynch-syndrome families. Int J Clin Exp Pathol. 2014;7:7297-303.
- [Google Scholar]
- Impact of microsatellite instability (MSI) on survival in high grade endometrial carcinoma. Gynecol Oncol. 2009;113:153-8.
- [CrossRef] [PubMed] [Google Scholar]
- Committee on Practice Bulletins-gynecology; Society of Gynecologic Oncology. ACOG practice bulletin No.147 Lynch syndrome. Obstet Gynecol. 2014;124:1042-54.
- [CrossRef] [PubMed] [Google Scholar]
- Germline MLH1 mutations are frequently identified in lynch syndrome patients with colorectal and endometrial carcinoma demonstrating isolated loss of PMS2 immunohistochemical expression. Am J Surg Pathol. 2015;39:1114-20.
- [CrossRef] [PubMed] [Google Scholar]
- Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23:276-92.
- [CrossRef] [PubMed] [Google Scholar]
- Integrated genomic characterization of endometrial carcinoma: The cancer genome atlas research network. Nature. 2013;497:67-73.
- [CrossRef] [PubMed] [Google Scholar]
- Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for lynch syndrome screening in endometrial cancers from GOG210: An NRG oncology and gynecologic oncology group study. J Clin Oncol. 2015;33:4301-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical challenged associated with universal screening for lynch syndrome-associated endomerial cancer. Cancer Prev Res. 2017;10:108-15.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecol Oncol. 2009;114:486-90.
- [CrossRef] [PubMed] [Google Scholar]
- Microsatellite instability in endometrial carcinomas: Clinicopathologic correlations in a series of 42 cases. Hum Pathol. 1998;29:1160-4.
- [CrossRef] [Google Scholar]
- DNA mismatch repair deficiency in endometrial carcinoma. Int J Gynecol Pathol. 2009;28:239-55.
- [CrossRef] [PubMed] [Google Scholar]
- Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007;25:2042-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association of tumor morphology with mismatch-repair protein status in older endometrial cancer patients. Implications for universal versus selective screening strategies for lynch syndrome. Am J Surg Pathol. 2014;38:793-800.
- [CrossRef] [PubMed] [Google Scholar]
- Society of gynecologic oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136:3-7.
- [CrossRef] [PubMed] [Google Scholar]
- One size may not fit all: The debate of universal tumor testing for lynch syndrome. Gynecol Oncol. 2015;137:2-3.
- [CrossRef] [PubMed] [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology. 2019. Uterine Neoplasms Screening Version 1. 2020, National Comprehensive Cancer Network. Available from: http://www.nccn.org [Last accessed 2020 Mar]
- [Google Scholar]
- Screening for lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mismatch repair protein expression in 1049 endometrial carcinomas, associations with body mass index, and other clinicopathologic variables. Gynecol Oncol. 2014;133:43-7.
- [CrossRef] [PubMed] [Google Scholar]
- Microsatellite instability in endometrial carcinomas: Frequent replication errors in tumors of early onset and/or of poorly differentiated type. Genes Chromosomes Cancer. 1995;14:128-32.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective evaluation of molecular screening for lynch syndrome in patients with endometrial cancer </=70 years. Gynecol Oncol. 2012;125:414-20.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol. 2015;137:306-10.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol. 2007;31:744-51.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of mismatch repair gene mutations in young patients with colorectal cancer and in patients with multiple tumours associated with hereditary non-polyposis colorectal cancer. Gut. 2006;55:1781-8.
- [CrossRef] [PubMed] [Google Scholar]







