Translate this page into:
CSF rhinorrhea following alectinib treatment for anaplastic lymphoma kinase rearranged lung adenocarcinoma - e contrario

*Corresponding author: Navneet Singh, Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh, India. navneetchd@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shrestha D, Gandra RR, Virk RS, Singh P, Mehta A, Kumar R, et al. CSF rhinorrhea following alectinib treatment for anaplastic lymphoma kinase rearranged lung adenocarcinoma - e contrario. Int J Mol Immuno Oncol 2020;5(3):127-30.
Abstract
Cerebrospinal fluid (CSF) rhinorrhea is a condition characterized by leakage of CSF from skull base through the nostril(s). It is commonly associated with trauma, surgery, infections of paranasal sinuses/skull base, and intracranial and skull base tumors. Among malignant causes, lung cancer is rarely associated with CSF rhinorrhea. Herein, we report the case of a 51-year-old lady who was initially diagnosed with metastatic lung adenocarcinoma (LUAC) with anaplastic lymphoma kinase (ALK) rearrangement and initiated on treatment with alectinib. She had good clinicoradiological response, but on follow-up developed CSF rhinorrhea that required surgical correction. We also discuss the proposed mechanisms associated with occurrence of CSF rhinorrhea in the setting of metastatic ALK-rearranged LUAC.
Keywords
Cerebrospinal fluid rhinorrhea
Alectinib
Anaplastic lymphoma kinase
Metastatic
Lung adenocarcinoma
INTRODUCTION
Cerebrospinal fluid (CSF) rhinorrhea is a well-known entity occurring after non-surgical treatment of pituitary tumors and meningiomas. However, it is extremely uncommon in lung cancer. In this manuscript, we describe the first reported case of CSF rhinorrhea occurring in a lady with anaplastic lymphoma kinase (ALK) rearranged metastatic non-small cell lung cancer (NSCLC) who had been on treatment with a second-generation ALK inhibitor for more than a year and was clinically and radiologically in partial remission. The postulated mechanisms as well as management of this condition are also described herein.
CASE PRESENTATION
A 51-year-old lady underwent evaluation for new-onset dyspnea in September 2018. Imaging revealed left lung hilar mass, pleuropericardial effusion, single 7 × 6 mm lesion in the right parietal white matter and multiple skeletal metastases (including right petroclival region) [Figure 1]. Bronchoscopic lung biopsy confirmed TTF-1-positive lung adenocarcinoma (LUAC). Immunochemistry with D5F3 showed ALK rearrangement. She was initiated on alectinib and denosumab. Repeat imaging after 3 months of treatment showed partial response (PR) with resolution of effusions and reduction in size of lung and brain lesions. The patient continued to derive symptomatic benefit with alectinib and sustained PR was documented on follow-up [Figure 2].
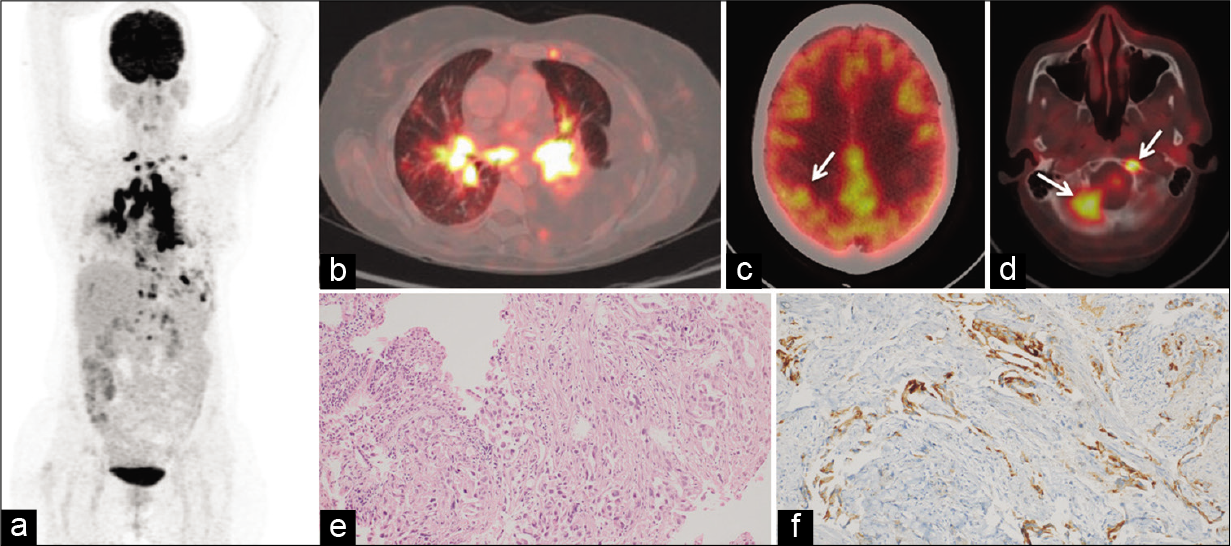
- Baseline positron emission tomography-computed tomography (PET-CT) scan (a) Maximum intensity projection (MIP) image (b) FDG- avid mediastinal lymph nodes and left hilar mass (c) FDG-avid solitary lesion in the right parietal lobe (white arrow) (d) FDG-avid lesions in the right petroclival and left anterior basiocciput regions of skull base (white arrows) (e) photomicrographs of bronchoscopic lung biopsy showing tumor cells arranged in glandular pattern in subepithelium and surrounded by desmoplastic stroma (hematoxylin and eosin stain, ×200) (f) Tumor cells showing strong cytoplasmic positivity for anaplastic lymphoma kinase by immunohistochemistry (D5F3 clone, Roche® Ventana®, ×200).
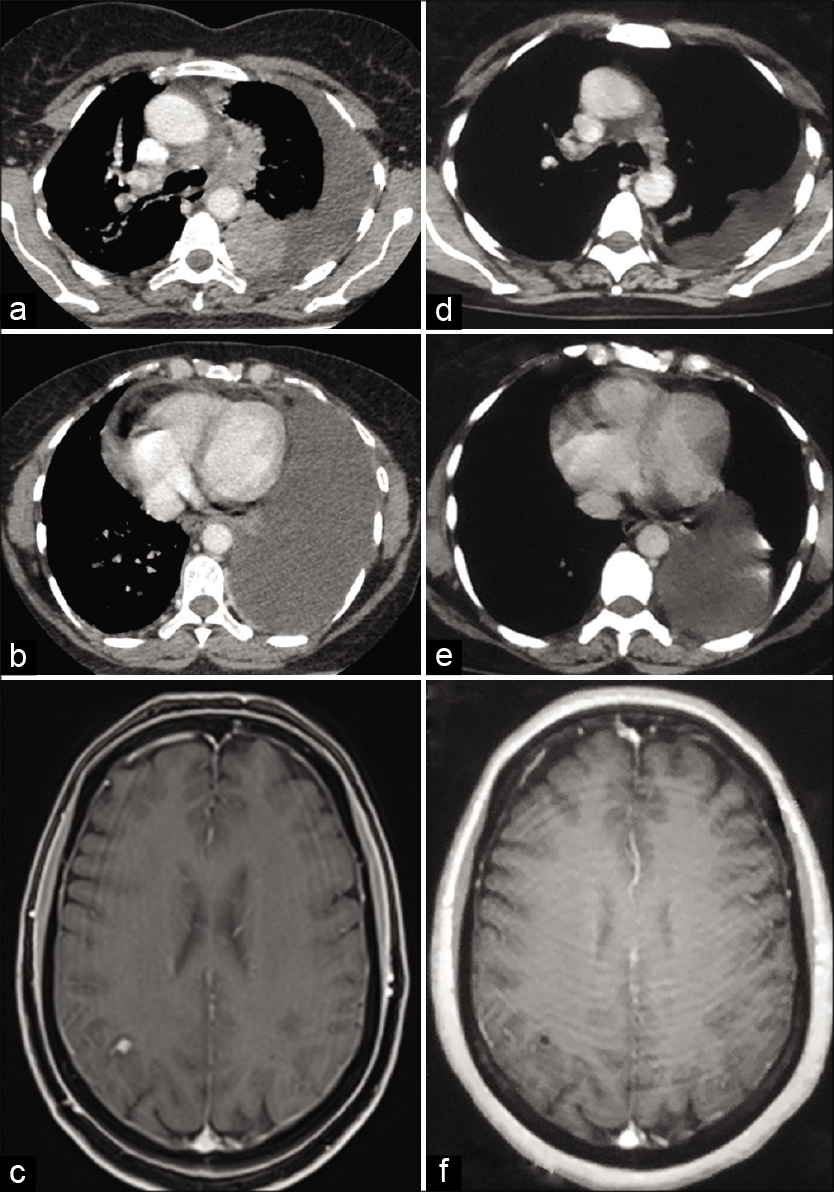
- Baseline images (before initiation of alectinib treatment) (a and b) contrast-enhanced axial computed tomography (CECT) scan of thorax showing left hilar mass, mediastinal lymphadenopathy, left-sided pleural effusion, and pericardial effusion and (c) Axial T1-weighted fat saturated post-gadolinium magnetic resonance image (MRI) of the brain showing 7 × 6 mm lesion in the right parietal lobe. Post-treatment images (following alectinib therapy) (d and e) CECT scan of thorax showing partial resolution of the left hilar mass, lymphadenopathy, and pleuropericardial effusion and (f) axial T1-weighted post-gadolinium MRI of brain showing resolution of the right parietal lobe lesion.
In February 2020, she was admitted for evaluation of clear watery discharge from the left nasal cavity which aggravated on stooping forward. There was no history of trauma. Magnetic resonance cisternography showed fluid in the left ethmoid with a defect in its roof. CEMRI brain showed an empty sella with flattening of posterior globe and perineural enhancement of the optic nerves. Ophthalmological evaluation revealed best-corrected visual acuity of 6/6 in both eyes. Fundoscopy and optic disk retinal nerve fiber layer optical coherence tomography showed no evidence of papilledema. The patient was taken up for surgical treatment of cerebrospinal (CSF) rhinorrhea. Intraoperatively, two defects with meningoceles were visualized in the left cribriform plate that was tackled with plasma-mediated ablation and endoscopic endonasal repair of defect performed with fat and vascularized nasoseptal flap [Figure 3]. She had complete recovery in the post-operative period with no recurrence and has been symptom free for the past 8 weeks.
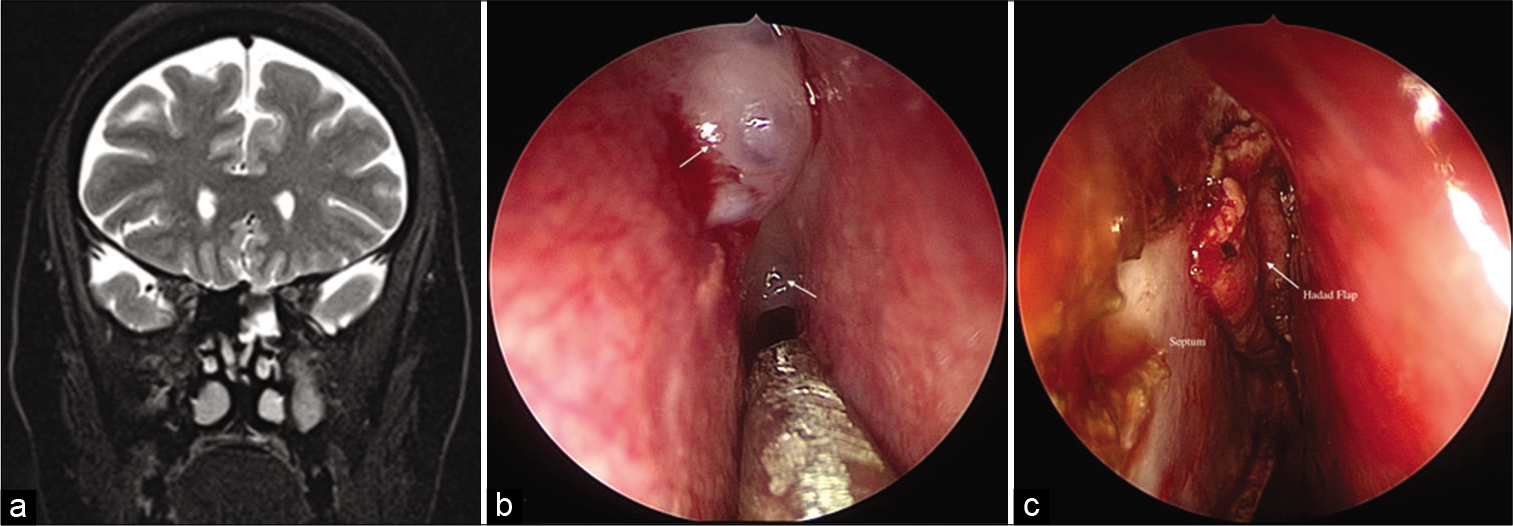
- (a) Coronal T2-weighted magnetic resonance images showing contiguous fluid signal intensity filling the left ethmoid with a defect in its roof. (b) Intraoperative image showing two defects with meningoceles (white arrows) in the cribriform plate. (c) Intraoperative image showing repair of the defects with fat and vascularized nasoseptal (Hadad) flap (white arrow) after plasma-mediated ablation of meningoceles.
DISCUSSION
CSF rhinorrhea has been described following non-surgical treatment (medical management/SBRT) of pituitary tumors and meningiomas.[1,2] To the best of our knowledge, there is a single published case report of CSF rhinorrhea occurring in metastatic lung cancer (EGFR mutated LUAC) after erlotinib treatment.[3]
Although the exact mechanism remains unclear, the possibility entertained in our case was something akin to benign intracranial hypertension (subclinical raised ICT), as there was empty sella sign with flattening of posterior globe and perineural enhancement of the optic nerves on imaging, and she had developed CSF rhinorrhea after 12 months of treatment initiation. However, alectinib is not known to be associated with this phenomenon. In ALEX and J-ALEX trials, visual disturbances (<2%) and headache (5%) have been described but no reports of benign intracranial hypertension or CSF rhinorrhea.[4,5]
The other postulated mechanism is rapid tumor regression after treatment initiation and unblocking of previously formed fistulous communication. Initially (treatment naïve setting), the metastatic tumor deposits erode into dura and bone of skull base leading to a “potential” CSF fistulous track that is not manifested clinically as it is held back by tumor deposits. However, rapid regression of these metastatic tumor deposits leads to “unplugging” of the fistulous site(s) manifesting clinically as CSF rhinorrhea. In the previous case report of EGFR mutated LUAC also, significant radiological response in skull base metastasis was demonstrated with treatment initiation.[3] In our case of ALK-rearranged LUAC, rapid clinical and radiological response was expected with alectinib – a second-generation ALK inhibitor with excellent brain penetration. In fact, repeat MRI showing marked reduction in size of brain parenchymal lesion obviated the need for stereotactic radio surgery. Furthermore, there was no demonstrable skeletal metastasis at skull base post- treatment.
However, the right petroclival metastasis seen in baseline radiology could not explain the CSF rhinorrhea in our case by the above-mentioned mechanism as the anatomical defect seen in MRI was on the left ethmoid roof. Furthermore, the intraoperative defect was seen in the left cribriform plate.
Due to the absence of common etiologies for CSF rhinorrhea, namely, trauma, post-surgical state, infection of PNS, congenital meningocele, etc., as well as lack of any other secondary etiologies for raised intracranial hypertension, the most plausible explanation of CSF rhinorrhea in our case could be idiopathic intracranial hypertension. The radiological findings favor raised ICT. The possibility of alectinib being an agent causing raised ICT needs further observation and evaluation. However, with very few reported cases of CSF rhinorrhea associated with lung cancer, it may be a difficult task to pinpoint the exact etiology in our case.
CONCLUSION
We believe that ours is the first reported case of CSF rhinorrhea in ALK-rearranged non-small cell lung cancer. It followed alectinib treatment although the causality of this association remains unproven.
Learning points for clinicians
This manuscript describes the first reported case of CSF rhinorrhea occurring in a lady with ALK-rearranged metastatic non-small cell lung cancers
It occurred while the patient had been on alectinib for more than a year and was clinically and radiologically in partial remission
Surgical treatment of the CSF leak led to complete resolution of symptoms
The proposed mechanism for this includes occurrence of a benign intracranial hypertension like syndrome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Medically induced CSF rhinorrhea following treatment of macroprolactinoma: Case series and literature review. Pituitary. 2018;21:561-70.
- [CrossRef] [PubMed] [Google Scholar]
- Dramatic radiographic response resulting in cerebrospinal fluid rhinorrhea associated with sunitinib therapy in recurrent atypical meningioma: Case report. J Neurosurg. 2016;127:965-70.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebrospinal fluid leak rhinorrhea after systemic erlotinib chemotherapy for metastatic lung cancer: A familiar problem from an unfamiliar culprit. World Neurosurg. 2017;108:992.e11-4.
- [CrossRef] [PubMed] [Google Scholar]
- Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829-38.
- [CrossRef] [PubMed] [Google Scholar]
- J-ALEX trial will crown alectinib as the standard choice for anaplastic lymphoma kinase positive untreated non-small cell lung cancer patients? J Thorac Dis. 2018;10:106-8.
- [CrossRef] [PubMed] [Google Scholar]






